930601
VH032-cyclopropane-F
≥95%
Synonym(s):
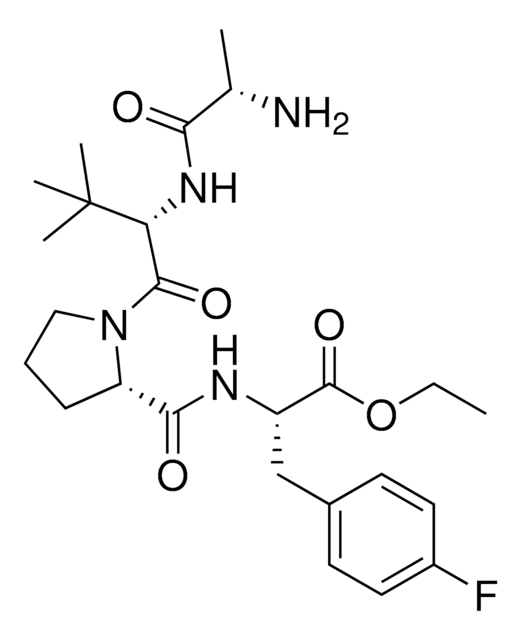
(S,R,S)-AHPC-cyclopropane-F, (2S,4R)-1-((S)-2-(1-Fluorocyclopropane-1-carboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(2-hydroxy-4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, (2S,4R)-1-[(S)-2-(1-Fluorocyclopropan-1-ylcarbonylamino)-3,3-dimethylbutanoyl]-4-hydroxy-N-[2-hydroxy-4-(4-methylthiazol-5-yl)benzyl]pyrrolidine-2-carboxamide, N-[(1-fluorocyclopropyl)carbonyl]-3-methyl-L-valyl-4-hydroxy-N-[[2-hydroxy-4-(4-methyl-5-thiazolyl)phenyl]methyl]-, (4R)-, L-Prolinamide
About This Item
Recommended Products
ligand
VH032
Quality Level
Assay
≥95%
form
powder
reaction suitability
reagent type: ligand
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
C([C@@H](NC(=O)C1(F)CC1)[C@@](C)(C)C)(=O)N2[C@H](C(NCC3=C(O)C=C(C=C3)C4=C(C)N=CS4)=O)C[C@@H](O)C2
Looking for similar products? Visit Product Comparison Guide
Application
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG3
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service