912034
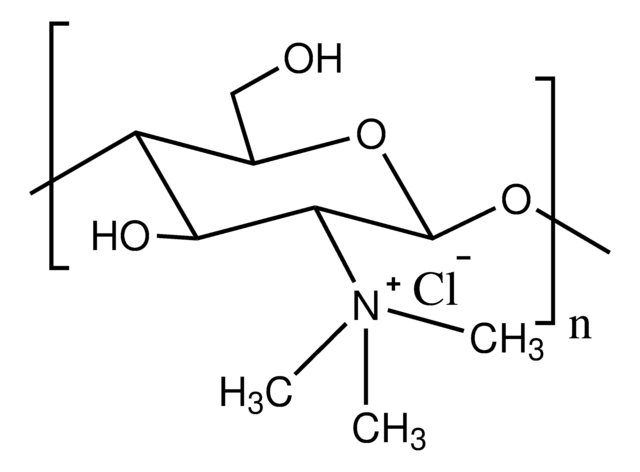
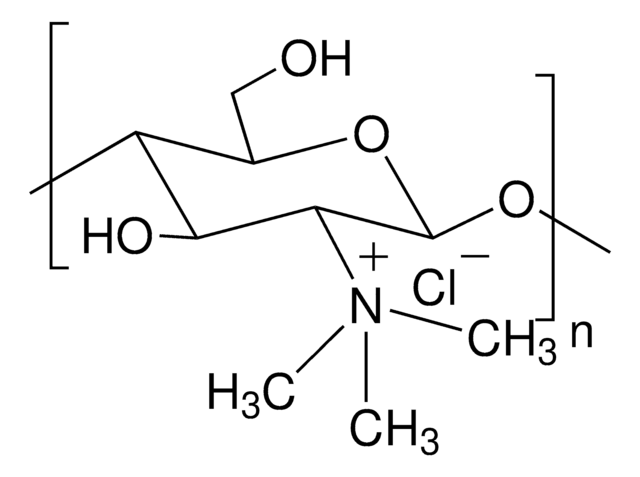
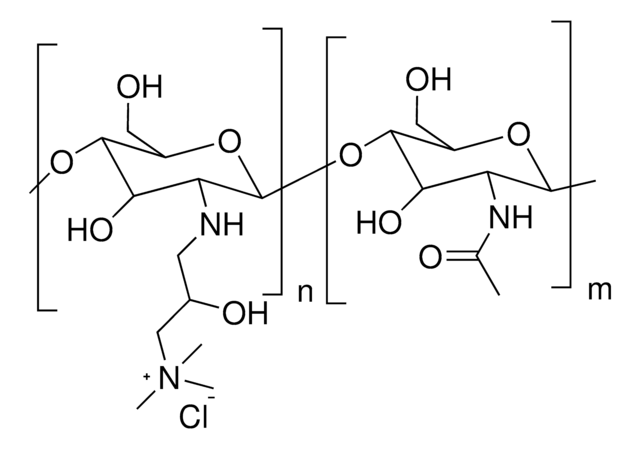
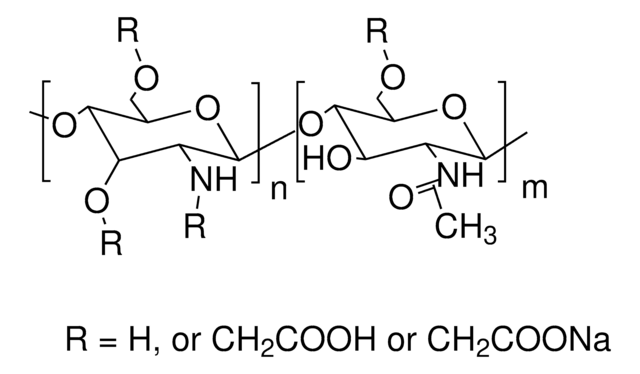
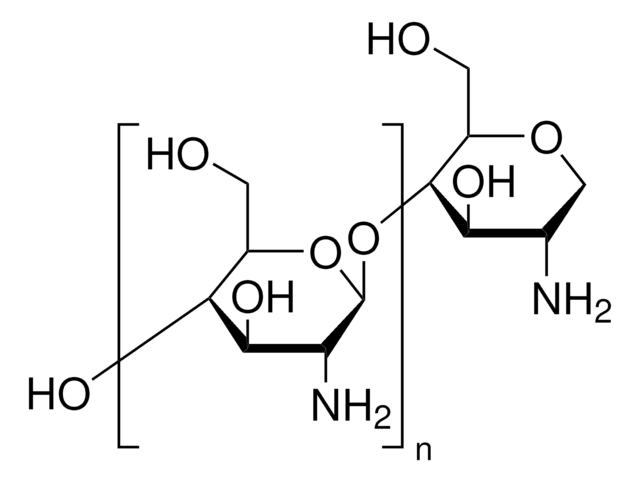
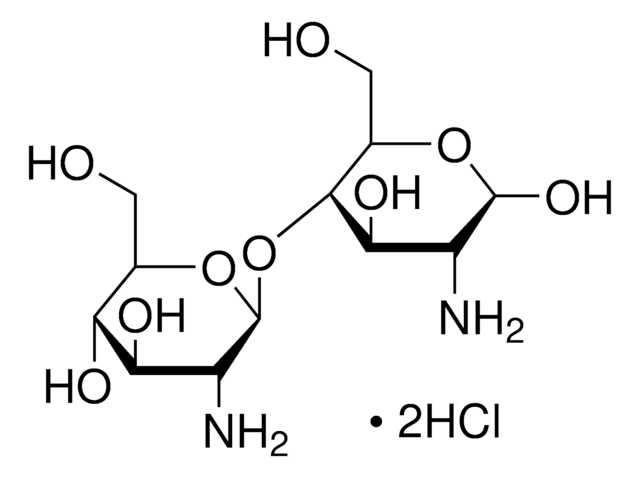
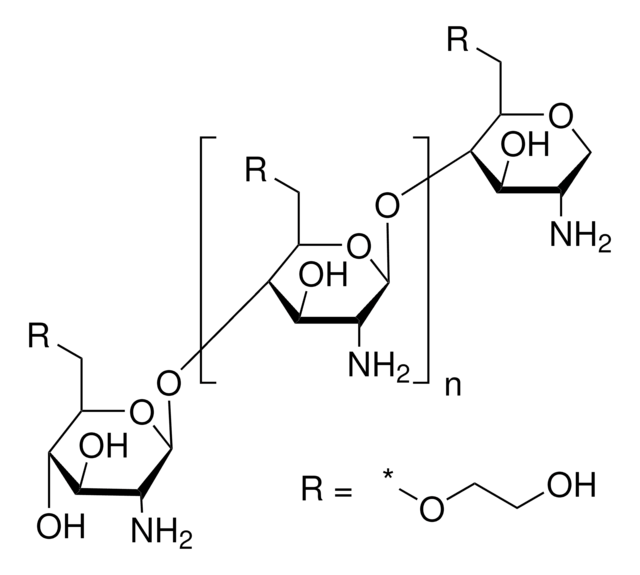
Trimethyl chitosan
high molecular weight, degree of quaternization 30-70%
Synonym(s):
N,N,N-Trimethyl chitosan, Chitosan trimethyl, Mucoadhesive polymers, TMC Chitosan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Looking for similar products? Visit Product Comparison Guide
Application
Chitosan offers remarkable biological properties, it has been widely used in drug delivery and tissue engineering applications. Chitosan has good mucoadhesive properties due to its positive charge, which increases the adhesion to mucosa and facilitates drug penetration. Also, chitosan possesses hemostatic properties, which makes it a good candidate for wound dressing applications.
The quaternization of the primary amine increases the water solubility of chitosan and keeps chitosan soluble over a wide pH range. Among all the quaternized chitosans, N, N, N-trimethyl chitosan chloride (TMC) is the most widely applied in biomedical applications.
The quaternization of the primary amine increases the water solubility of chitosan and keeps chitosan soluble over a wide pH range. Among all the quaternized chitosans, N, N, N-trimethyl chitosan chloride (TMC) is the most widely applied in biomedical applications.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rajesh K Kainthan et al.
Biomaterials, 29(11), 1693-1704 (2008-01-16)
There is a huge clinical demand for Human Serum Albumin (HSA), with a world market of approximately $1.5B/year. Concern over prion and viral transmission in the blood supply has led to a need for safer substitutes and offers the opportunity
Preparation and Characterization of Particles from Chitosan with Different Molecular Weights and Their Trimethyl Chitosan Derivatives for Nasal Immunization.
Boonyo W, et al.
Journal of Metals, Materials and Minerals, 18(2), 59-65 (2008)
Rajesh Kumar Kainthan et al.
Biomaterials, 27(31), 5377-5390 (2006-07-21)
A novel class of hyperbranched polymers based on polyglycerol (PG) and poly(ethylene glycol) (PEG) are synthesized by multibranching anionic ring opening polymerization. Multivalent cationic sites are added to these polymers by a post-amination and quarternization reactions. Blood compatibility studies using
Preparation and NMR characterization of highly substituted IV-trimethyl chitosan chloride.
Sieval A B, et al.
Carbohydrate Polymers, 36, 157-165 (1998)
Rajesh Kumar Kainthan et al.
Biomacromolecules, 9(3), 886-895 (2008-02-06)
This paper discusses the binding and release properties of hydrophobically modified hyperbranched polyglycerol-polyethylene glycol copolymers that were originally developed as human serum albumin (HSA) substitutes. Their unimolecular micellar nature in aqueous solution has been proven by size measurements and other
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service