711772
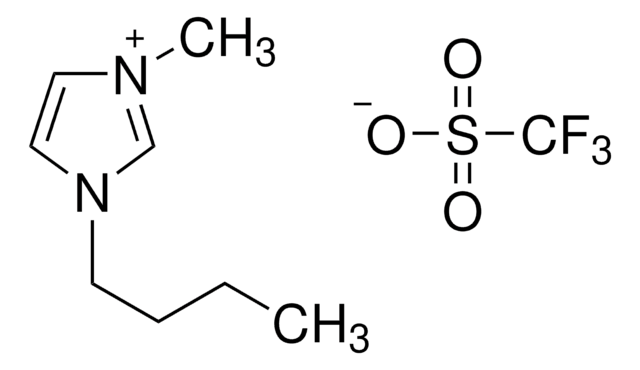
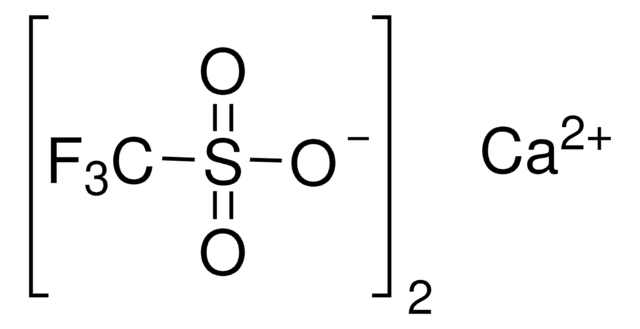
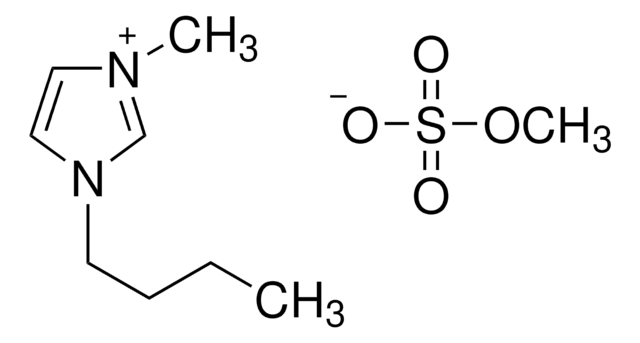
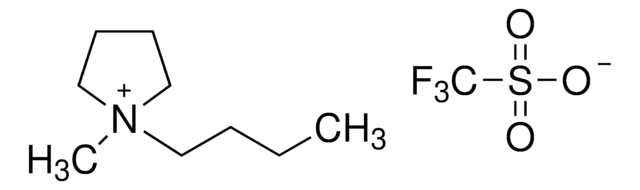
1-Butyl-3-methylimidazolium trifluoromethanesulfonate
97%
Synonym(s):
BMIM Otf
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H15F3N2O3S
CAS Number:
Molecular Weight:
288.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
impurities
≤0.5% water
refractive index
n20/D 1.434
density
1.292 g/mL at 20 °C (lit.)
SMILES string
[O-]S(=O)(=O)C(F)(F)F.CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.CHF3O3S/c1-3-4-5-10-7-6-9(2)8-10;2-1(3,4)8(5,6)7/h6-8H,3-5H2,1-2H3;(H,5,6,7)/q+1;/p-1
InChI key
FRZPYEHDSAQGAS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
1-Butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][OTf] is a versatile ionic liquid commonly used in synthetic chemistry.
Application
[bmim][OTf] can be used as:
- A glycosylation promoter in the synthesis of oligosaccharides from thiophenyl and trichloroacetimidate glycoside donors.
- A solvent for the extraction of sulfur and nitrogen containing aromatic organic compounds from aliphatic hydrocarbons.
- A reagent in the preparation of ion-conductive polymeric membranes.
- A dopant in the synthesis of poly (ethyl methacrylate) based polymer electrolytes to increase the ionic conductivity.
- A reaction medium to synthesize aryl ketones by Friedel–Crafts benzoylation of aryl compounds by using bismuth triflate as a catalyst.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>212.0 °F
Flash Point(C)
> 100 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient Air?Stable Organometallic Low?Molecular?Mass Gelators for Ionic Liquids: Synthesis, Aggregation and Application of Pyridine?Bridged Bis (benzimidazolylidene)?Palladium Complexes.
Tu T, et al.
Chemistry?A European Journal, 15(8), 1853-1861 (2009)
[bmim][OTf]: a versatile room temperature glycosylation promoter.
Galan M C, et al.
Tetrahedron Letters, 50(4), 442-445 (2009)
A new application of Baylis?Hillman alcohols to a diastereoselective synthesis of 3-nitrothietanes.
Rai A and Yadav L D S
Tetrahedron, 68(11), 2459-2464 (2012)
Selective Quenching of 2-Naphtholate Fluorescence by Imidazolium Ionic Liquids.
Kumar V and Pandey S
The Journal of Physical Chemistry B, 116(39), 12030-12037 (2012)
tert-Amyl ethyl ether separation from its mixtures with ethanol using the 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid: liquid- liquid equilibrium
Arce A, et al.
Industrial & Engineering Chemistry Research, 43(26), 8323-8327 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service