All Photos(1)
About This Item
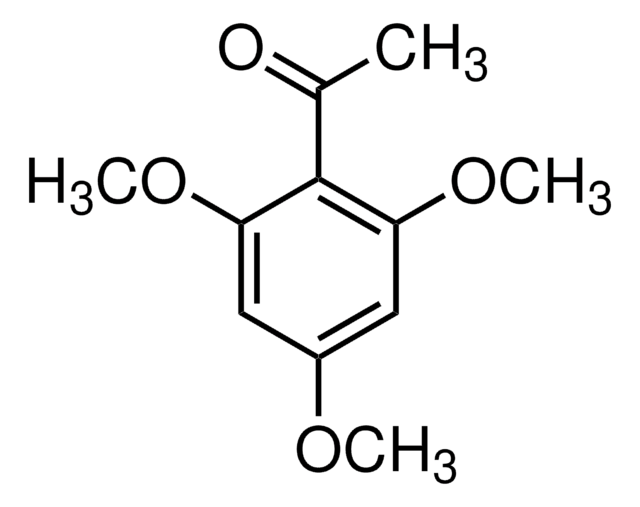
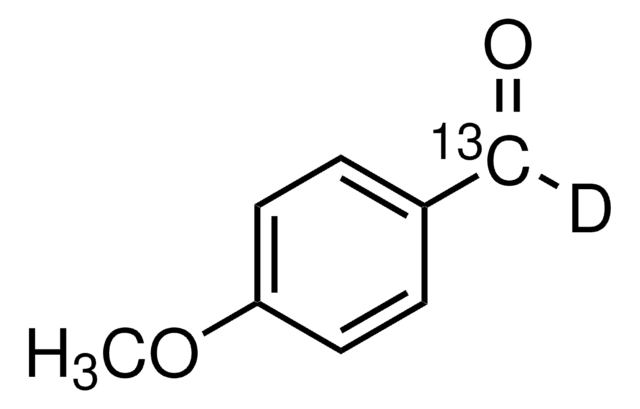
Empirical Formula (Hill Notation):
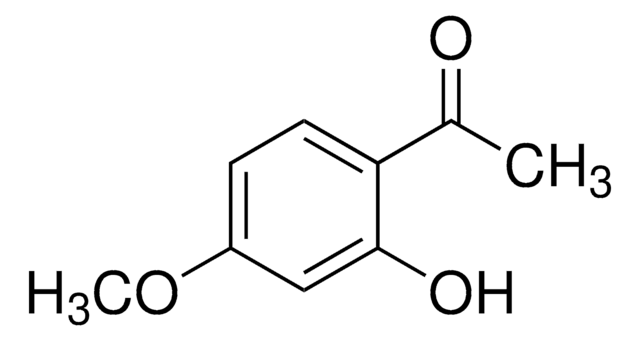
C10H12O4
CAS Number:
Molecular Weight:
196.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
Assay:
97%
Recommended Products
Quality Level
Assay
97%
form
solid
mp
80-84 °C (lit.)
functional group
ketone
SMILES string
COc1cc(O)c(C(C)=O)c(OC)c1
InChI
1S/C10H12O4/c1-6(11)10-8(12)4-7(13-2)5-9(10)14-3/h4-5,12H,1-3H3
InChI key
FBUBVLUPUDBFME-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T R Pinheiro et al.
Arzneimittel-Forschung, 49(12), 1039-1043 (2000-01-15)
This study describes the fungistatic effect of xanthoxyline (CAS 90-24-4) and its derivatives against a panel of yeasts, filamentous fungi and dermatophytes, by using the agar dilution method. Results indicated that simple structural modifications led to more potent derivatives, especially
Rodrigo dos Santos et al.
Archiv der Pharmazie, 339(5), 227-237 (2006-03-31)
Semi-empirical molecular orbital calculations at AM1 level were done with the aim to investigate the structure-activity relationships of antispasmodic activities of ten 2-(X-benzyloxy)-4,6-dimethoxyacetophenones with X = H, 4'-F, 4'-NO2, 4'-CH3, 4'-Cl, 3',4'-(CH3)2, 4'-OCH3, 4'-Br, 4'-OCH2C6H5, and 4'-C(CH3)3, against acetylcholine-induced contraction
Paula Boeck et al.
Bioorganic & medicinal chemistry, 14(5), 1538-1545 (2006-01-03)
Eighteen analogues of an active natural chalcone were synthesized using xanthoxyline and some derivatives, and these analogues were tested for selective activity against both promastigotes and intracellular amastigotes of Leishmania amazonensis in vitro. Three analogues (10, 12, and 19) containing
Fátima de Campos-Buzzi et al.
Archiv der Pharmazie, 339(7), 361-365 (2006-07-14)
A wide variety of noxious stimuli are known to induce a sensation of pain evoked in a remote region of the body. Here, we show that chalcones can inhibit the pain provoked by the administration of an intraperitoneal acetic acid
Z R Vaz et al.
The Journal of pharmacology and experimental therapeutics, 278(1), 304-312 (1996-07-01)
The antinociceptive effect of the novel xanthoxyline derivative 2-(4-bromobenzoyl)-3-methyl-4-6-dimethoxy benzofuran) (BMDB), given i.p., p.o., s.c., subplantarly, intrathecally or by i.c.v. routes was assessed in five models of chemical and thermal nociception in mice, namely acetic acid-induced abdominal constriction, formalin and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service