All Photos(1)
About This Item
Empirical Formula (Hill Notation):
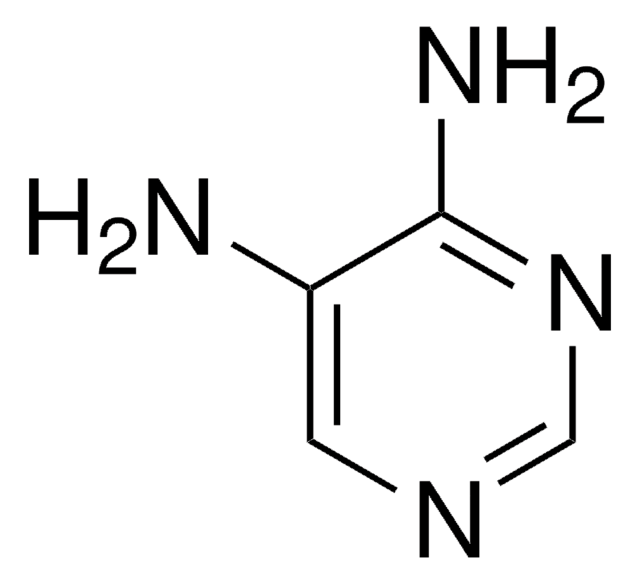
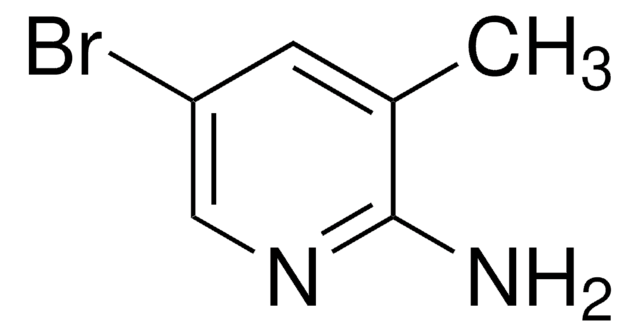
C5H6BrN3
CAS Number:
Molecular Weight:
188.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
155 °C (dec.) (lit.)
functional group
bromo
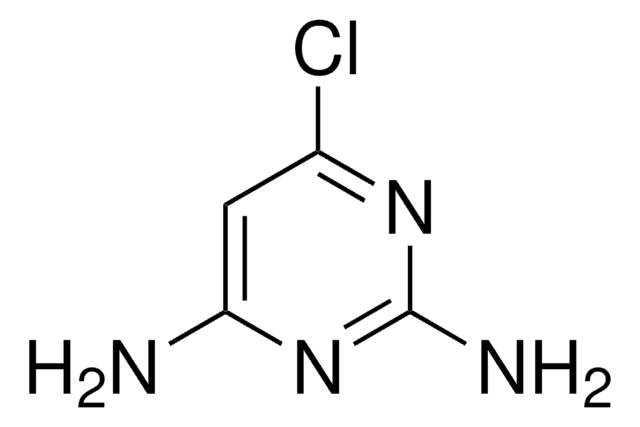
SMILES string
Nc1cc(Br)cnc1N
InChI
1S/C5H6BrN3/c6-3-1-4(7)5(8)9-2-3/h1-2H,7H2,(H2,8,9)
InChI key
YRGMYJUKFJPNPD-UHFFFAOYSA-N
General description
2,3-Diamino-5-bromopyridine can be prepared from 2-amino-3-nitro-5-bromopyridine via reduction using stannous chloride.
Application
2,3-Diamino-5-bromopyridine may be used in the preparation of the following heterocyclic compounds:
- 6-bromoimidazo-[b]pyridine
- 11-bromopyrido[2′,3′:5,6]pyrazino[2,3-f][1,10]phenanthroline
- 6-bromo-3-(tetrahydro-2H-pyran-2-yl)-3H-imidazo[4,5-b]pyridine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Microwave-Assisted C-2 Direct Alkenylation of Imidazo [4, 5-b] pyridines: Access to Fluorescent Purine Isosteres with Remarkably Large Stokes Shifts.
Baladi T, et al.
European Journal of Organic Chemistry, 2016(14), 2421-2434 (2016)
Sundaram Ellairaja et al.
Biosensors & bioelectronics, 91, 82-88 (2016-12-20)
Bilirubin, a key biomarker for the jaundice and its clinical diagnosis needs a better analytical tool. A novel and simple fluorescent platform based on (2,2'-((1E,1'E)-((6-bromopyridine-2,3-diyl) bis(azanylylidene)) bis(methanylylidene diphenol) (BAMD) was designed. BAMD showed a remarkable fluorescent intensity with a very
Metabolite analogs. VIII. Syntheses of some imidazopyridines and pyridotriazoles.
Graboyes H and Day AR.
Journal of the American Chemical Society, 79(24), 6421-6426 (1957)
Re (I) Complexes of Substituted dppz: A Computational and Spectroscopic Study.
van der Salm H, et al.
Inorganic Chemistry, 53(6), 3126-3140 (2014)
Sheta M Sheta et al.
Dalton transactions (Cambridge, England : 2003), 47(14), 4847-4855 (2018-03-16)
Novel copper metal organic framework nanoparticles Cu-MOF-NPs (C1) were prepared via two simple alternative methods and confirmed by analytical characterization using mass, IR, Raman, XRD spectrum, HR-TEM and TGA-DSC. Mass spectroscopy revealed the molecular ion peak at 647 m/z for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service