All Photos(1)
About This Item
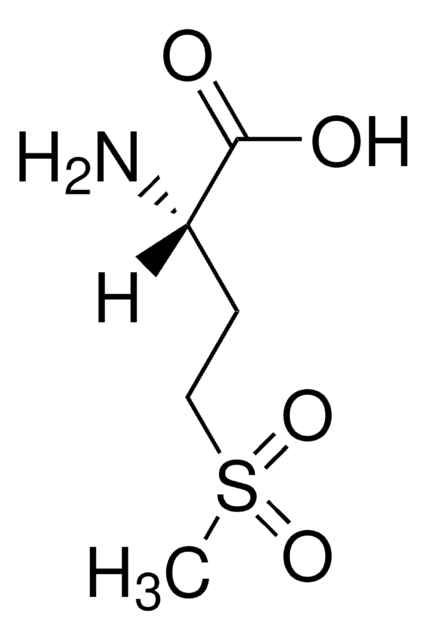
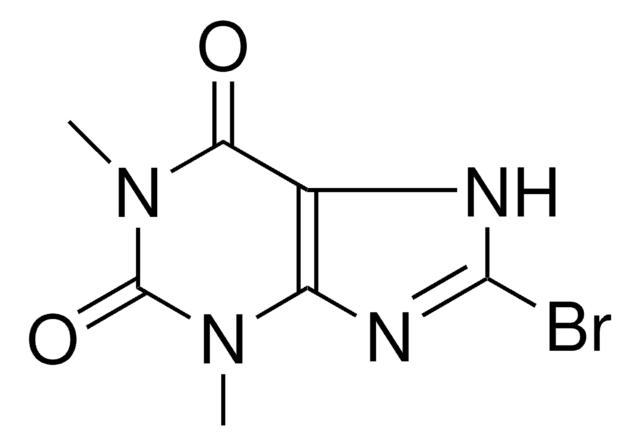
Empirical Formula (Hill Notation):
C5H3BrN4O
CAS Number:
Molecular Weight:
215.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
>350 °C (lit.)
functional group
bromo
SMILES string
BrC1=Nc2nc[nH]c2C(=O)N1
InChI
1S/C5H3BrN4O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H2,7,8,9,10,11)
InChI key
ONXCBJOMYNPZNI-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R S Sodum et al.
Chemical research in toxicology, 11(12), 1453-1459 (1998-12-22)
2-Nitropropane, an industrial chemical and a hepatocarcinogen in rats, induces aryl sulfotransferase-mediated liver DNA and RNA base modifications [Sodum, R. S., Sohn, O. S., Nie, G., and Fiala, E. S. (1994) Chem. Res. Toxicol. 7, 344-351]. Two of these modifications
H Xu et al.
Journal of medicinal chemistry, 38(1), 49-57 (1995-01-06)
Two series of selective inhibitors of herpes simplex virus types 1 and 2 (HSV1,2) thymidine kinases (TK) have been developed as potential treatment of recurrent virus infections. Among compounds related to the potent base analog N2-[m-(trifluoromethyl)phenyl]guanine (mCF3-PG), none was a
Sheng Ding et al.
Journal of combinatorial chemistry, 4(2), 183-186 (2002-03-12)
A resin-capture and release strategy for making combinatorial 2,6,9-trisubstituted purine libraries is demonstrated by capturing N9-derivatized purines at the C6 position with a thio-modified polymer. The C2 fluoro group is subsequently substituted with primary and secondary amines followed by thioether
M M Butler et al.
Nucleic acids research, 18(24), 7381-7387 (1990-12-25)
6-(p-Hydroxyphenylhydrazino)uracil (H2-HPUra) is a selective and potent inhibitor of the replication-specific class III DNA polymerase (pol III) of Gr+ bacteria. Although formally a pyrimidine, H2-HPUra derives its inhibitory activity from its specific capacity to mimic the purine nucleotide, dGTP. We
Andrzej Manikowski et al.
Journal of medicinal chemistry, 48(11), 3919-3929 (2005-05-27)
Derivatives of the herpes simplex thymidine kinase inhibitor HBPG [2-phenylamino-9-(4-hydroxybutyl)-6-oxopurine] have been synthesized and tested for inhibitory activity against recombinant enzymes (TK) from herpes simplex types 1 and 2 (HSV-1, HSV-2). The compounds inhibited phosphorylation of [3H]thymidine by both enzymes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service