All Photos(2)
About This Item
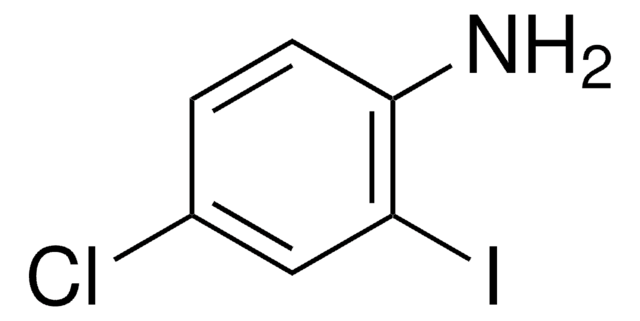
Linear Formula:
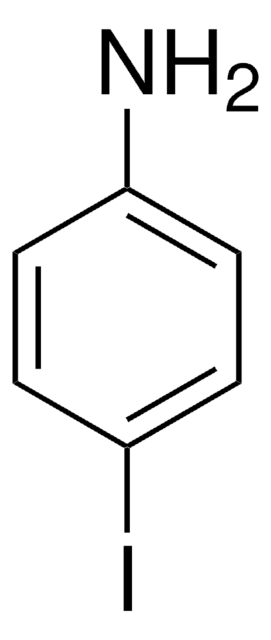
BrC6H3(I)NH2
CAS Number:
Molecular Weight:
297.92
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
69-72 °C (lit.)
functional group
bromo
iodo
SMILES string
Nc1ccc(Br)cc1I
InChI
1S/C6H5BrIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
InChI key
HHTYEQWCHQEJNV-UHFFFAOYSA-N
General description
4-Bromo-2-iodoaniline is a 2-iodoaniline derivative. It can be prepared by reacting 4-bromoaniline with iodine.
Application
4-Bromo-2-iodoaniline may be used in the following:
- Preparation of quinolone derivatives.
- Synthesis of a resin-bound sulfonamide, which was used as a starting material for the preparation of 2,3,5-trisubstituted indoles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Y Wu et al.
Organic letters, 3(24), 3827-3830 (2001-11-27)
2,3,5-Trisubstituted indoles are synthesized in three steps starting from resin-bound aniline 2. R1 is introduced by a palladium-mediated coupling of the aryl iodide with terminal alkynes followed by intramolecular cyclization to form the indole core. Acylation at C-3 with an
ON SOME HALOGEN DERIVATIVES OF AROMATIC AMINES AND THEIR ANALYSIS. I. 1.
Dains FB, et al.
Journal of the American Chemical Society, 40(6), 930-936 (1918)
Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines.
Cortese NA, et al.
The Journal of Organic Chemistry, 43(15), 2952-2958 (1978)
Shaei Huang et al.
Bioorganic & medicinal chemistry letters, 16(22), 5907-5912 (2006-09-23)
Through a comparison of X-ray co-crystallographic data for 1 and 2 in the Chek1 active site, it was hypothesized that the affinity of the indolylquinolinone series (2) for Chek1 kinase would be improved via C6 substitution into the hydrophobic region
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service