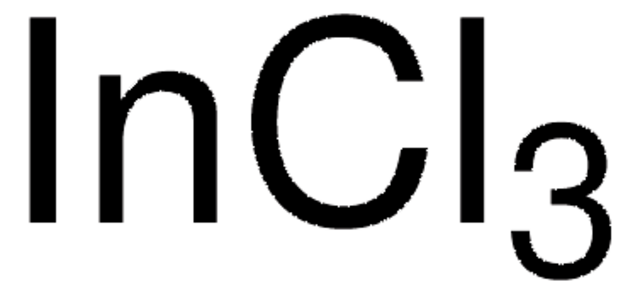510270
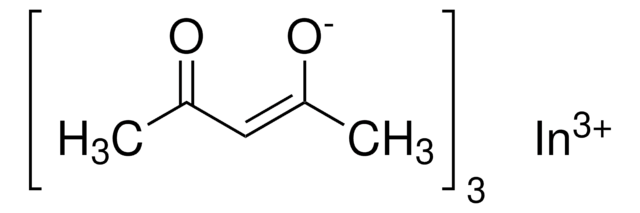
Indium(III) acetate
99.99% trace metals basis
Synonym(s):
Indium triacetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
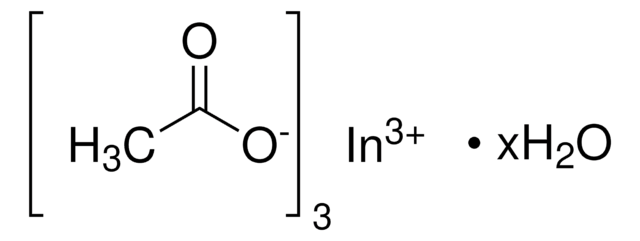
In(C2H3O2)3
CAS Number:
Molecular Weight:
291.95
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.99% trace metals basis
form
solid
reaction suitability
core: indium
reagent type: catalyst
mp
270 °C (dec.) (lit.)
SMILES string
CC(=O)O[In](OC(C)=O)OC(C)=O
InChI
1S/3C2H4O2.In/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3
InChI key
VBXWCGWXDOBUQZ-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Indium(III) acetate is a white crystalline water-soluble solid that decomposes to indium oxide upon heating. It is used as a catalyst in intermolecular and intramolecular acyl substitution reactions. It is also widely used as CVD precursor to prepare indium oxide thin oxide.
Application
Indium(III) acetate can be used:
- As a precursor to fabricate In2S3 thin films via chemical spray pyrolysis. These films are used as electron transport layers in highly efficient perovskite solar cells.
- To prepare indium arsenide quantum dots which are infrared emitting nanomaterials used in optoelectronic and biomedical applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics analysis for non-isothermal decomposition ?-irradiated indium acetate.
Al-Resayes SI, et al
Arabian Journal of Chemistry, 3(3), 191-194 (2010)
Characteristics of CuInS2/ZnS quantum dots and its application on LED
Kim H,et al
Journal of Crystal Growth, 326, 90-93 (2011)
Katsukiyo Miura et al.
Organic letters, 10(1), 133-136 (2007-12-13)
In the presence of phenylsilane and a catalytic amount of indium(III) acetate, organic iodides added to electron-deficient alkenes in ethanol at room temperature. Both simple and functionalized organic iodides were applicable to this reaction. A plausible reaction mechanism involves the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service