All Photos(2)
About This Item
Linear Formula:
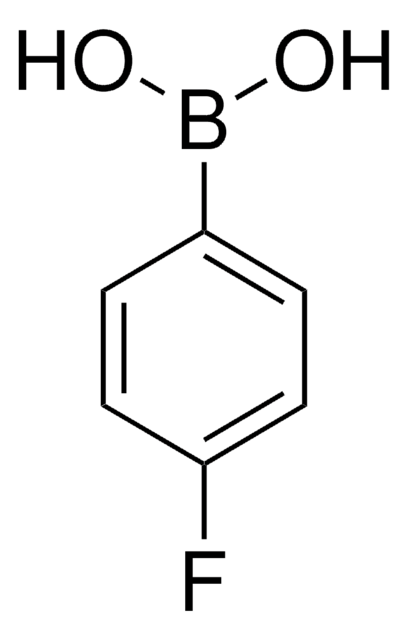
C6H5OC6H4B(OH)2
CAS Number:
Molecular Weight:
214.02
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
141-145 °C (lit.)
functional group
phenoxy
SMILES string
OB(O)c1ccc(Oc2ccccc2)cc1
InChI
1S/C12H11BO3/c14-13(15)10-6-8-12(9-7-10)16-11-4-2-1-3-5-11/h1-9,14-15H
InChI key
KFXUHRXGLWUOJT-UHFFFAOYSA-N
Related Categories
Application
4-Phenoxyphenylboronic acid can be used as a reactant:
- In the Suzuki-Miyaura coupling reaction to synthesize aryl derivatives via C-C bond formation by reacting with different aryl halides over a palladium catalyst.
- To prepare 1-phenoxy-4-(trifluoromethyl)benzene via oxidative trifluoromethylation using Chan-Lam-type reaction conditions.
- To synthesize evobrutinib, a potent Bruton′s tyrosine kinase (BTK) inhibitor.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Room temperature aryl trifluoromethylation via copper-mediated oxidative cross-coupling
Senecal TD, et al.
The Journal of Organic Chemistry, 76(4), 1174-1176 (2011)
Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models
Haselmayer P, et al.
Journal of Immunology, 202(10), 2888-2906 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service