All Photos(2)
About This Item
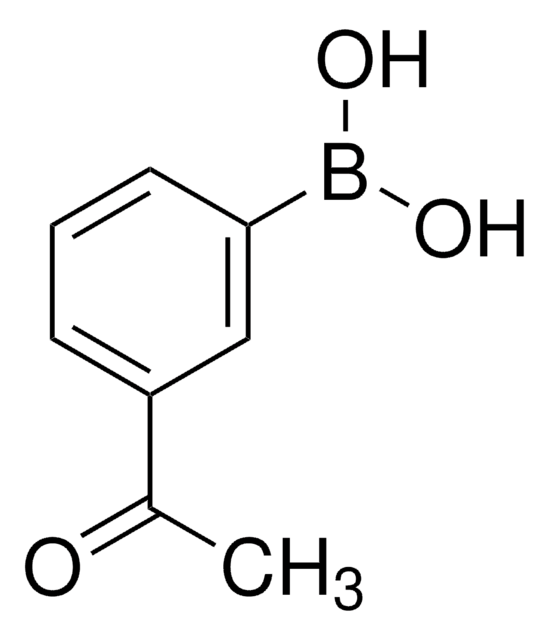
Linear Formula:
CH3COC6H4B(OH)2
CAS Number:
Molecular Weight:
163.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
240-244 °C (lit.)
SMILES string
CC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-2-4-8(5-3-7)9(11)12/h2-5,11-12H,1H3
InChI key
OBQRODBYVNIZJU-UHFFFAOYSA-N
Related Categories
General description
4-Acetylphenylboronic acid is a boronate, belongs to a class of synthetic organic compounds. It reacts rapidly with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives. It undergoes Suzuki coupling with 4-bromotriphenylamine catalyzed by dichlorobis(triphenylphosphine)Pd(II), during the synthesis of dendrimers.
Application
4-Acetylphenylboronic acid was used in the synthesis of 4′-azidoacetophenone.
Reactant involved in:
- Palladium-catalyzed decarboxylative coupling
- Copper-catalyzed hydroxylation
- Palladium-catalyzed Suzuki-Miyaura cross-coupling
- Cross-coupling with α-bromocarbonyl compounds
- Oxidation catalyzed by Baeyer-Villiger monooxygenases
- 1,5-substitution reactions
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kimberly D Grimes et al.
Synthesis, 2010(9), 1441-1448 (2010-06-08)
We report the copper(II)-catalyzed conversion of organoboron compounds into the corresponding azide derivatives. A systematic series of phenylboronic acid derivatives is evaluated to examine the importance of steric and electronic effects of the substituents on reaction yield as well as
Adam Sikora et al.
Free radical biology & medicine, 47(10), 1401-1407 (2009-08-19)
In this study, we show that boronates, a class of synthetic organic compounds, react rapidly and stoichiometrically with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives as major products. Using a stopped-flow kinetic technique, we measured the second-order rate constants for
A New Efficient Convergent Synthesis of Conjugated Aryl-containing Dendrimers.
El-Deeb IM and Lee SH.
Bull. Korean Chem. Soc., 31(6), 1757-1760 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![1-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/280/787/64aa2a50-1d44-4c16-ace9-c54ea40606e6/640/64aa2a50-1d44-4c16-ace9-c54ea40606e6.png)