All Photos(2)
About This Item
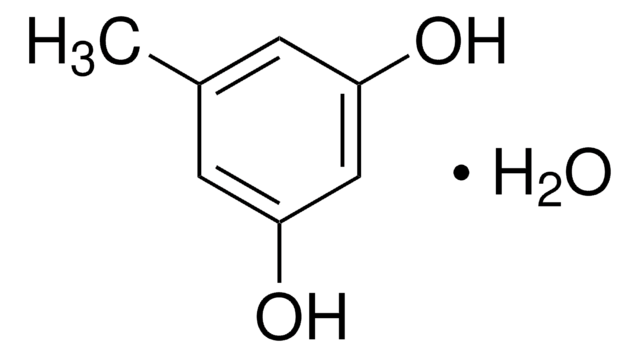
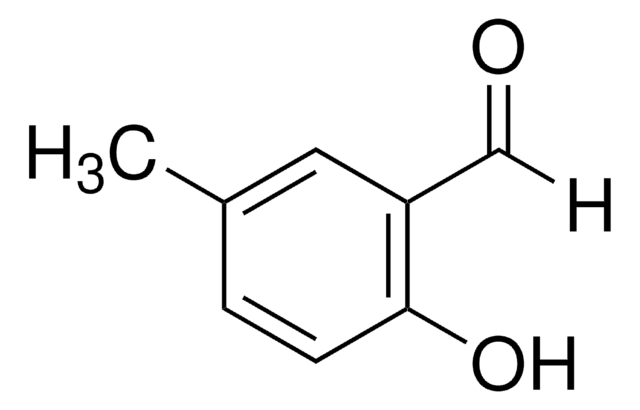
Linear Formula:
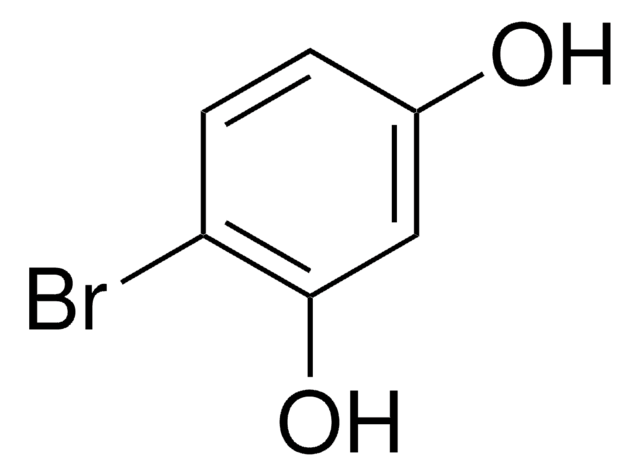
CH3C6H3-1,3-(OH)2
CAS Number:
Molecular Weight:
124.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
106-112 °C (lit.)
SMILES string
Cc1cc(O)cc(O)c1
InChI
1S/C7H8O2/c1-5-2-6(8)4-7(9)3-5/h2-4,8-9H,1H3
InChI key
OIPPWFOQEKKFEE-UHFFFAOYSA-N
Related Categories
Application
Orcinol can be used to synthesize:
- Orcinol-containing azacryptands for use in optical amplifiers and light-emitting devices.
- Ternary co-crystal with 4,4′-bipyridine.
- Low-density carbon aerogels in the presence of formaldehyde.
- PEG-orcinol coumarins with potent tyrosinase inhibitory activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karen J Marsh et al.
Ecology, 87(8), 2103-2112 (2006-08-30)
Most herbivores eat more and survive better when they have access to a variety of foods. One explanation involves the detoxification of plant secondary metabolites (PSMs). By feeding from a variety of plants that contain different classes of PSMs, animals
Shape and size mimicry in the design of ternary molecular solids: towards a robust strategy for crystal engineering
Tothadi S, et al.
Chemical Communications (Cambridge, England), 47(44), 12080-12082 (2011)
Mikkel Schultz-Johansen et al.
Frontiers in microbiology, 9, 839-839 (2018-05-19)
Marine microbes are a rich source of enzymes for the degradation of diverse polysaccharides. Paraglaciecola hydrolytica S66T is a marine bacterium capable of hydrolyzing polysaccharides found in the cell wall of red macroalgae. In this study, we applied an approach
PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol-coumarin derivatives: Preliminary evaluation of their inhibitory activity on mushroom tyrosinase
Tocco G, et al.
Bioorganic & Medicinal Chemistry Letters, 19(1), 36-39 (2009)
One-pot synthesis of new functionalized azacryptands from resorcinol derivatives for advanced photonic materials
Ka J-W and Kim HK
Tetrahedron Letters, 45(23), 4519-4523 (2004)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 447420-5G | 4061835176885 |
| 447420-25G | 4061832288567 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service