412805
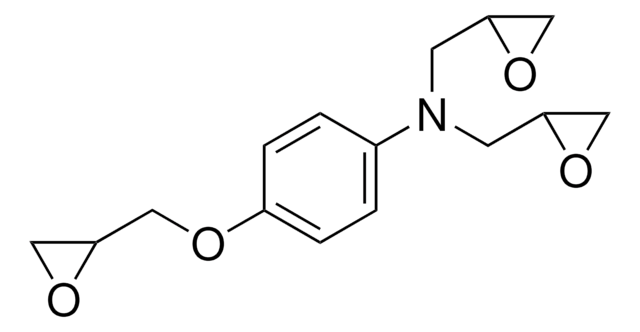
4,4′-Methylenebis(N,N-diglycidylaniline)
Synonym(s):
4,4′-Methylenebis[N ,N -bis(oxiranylmethyl)aniline], 4,4′-Methylenedianiline tetraglycidyl ether, N ,N ,N ′,N ′-Tetraglycidyl-4,4′-methylenebisbenzenamine, N ,N ,N ′,N ′-Tetraglycidyl-4,4′-methylenedianiline, Bis[4-(diglycidylamino)phenyl]methane
About This Item
Recommended Products
form
viscous liquid
refractive index
n20/D 1.601 (lit.)
density
1.15 g/mL at 25 °C (lit.)
SMILES string
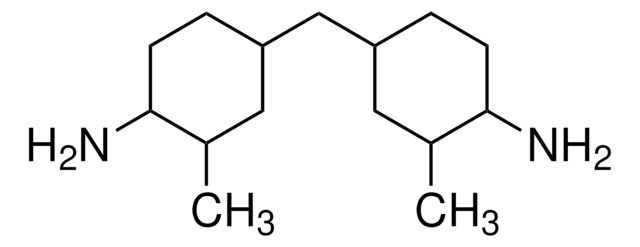
C1OC1CN(CC2CO2)c3ccc(Cc4ccc(cc4)N(CC5CO5)CC6CO6)cc3
InChI
1S/C25H30N2O4/c1-5-20(26(10-22-14-28-22)11-23-15-29-23)6-2-18(1)9-19-3-7-21(8-4-19)27(12-24-16-30-24)13-25-17-31-25/h1-8,22-25H,9-17H2
InChI key
FAUAZXVRLVIARB-UHFFFAOYSA-N
General description
Application
- As a starting material to synthesize UV-curable tetra-functional epoxy acrylate (EA4), which is used as a crosslinker for UV-curable resins.
- In the synthesis of bismaleimide/diallyl bisphenol A (BMI/DBA)–epoxy interpenetrating network resins, which have potential applications in the aerospace and automotive industries because of their high thermal stability and low activation energy.
- As a crosslinking agent in the production of biocompatible materials, such as hydrogels and other tissue engineering scaffolds. Its ability to form strong and stable crosslinks makes it valuable in the creation of medical devices, implants, and drug delivery systems. Additionally, it is utilized in the synthesis of biocompatible polymers and materials with tailored properties for biomedical applications.
- Poly(hexamethylene biguanide) based polymer networks which are applicable as catalysts for the transesterification of vegetable oils.
- Tetra-functional epoxy-acrylate UV curable resins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service