411760
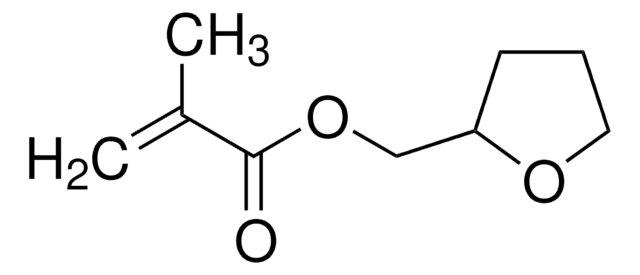
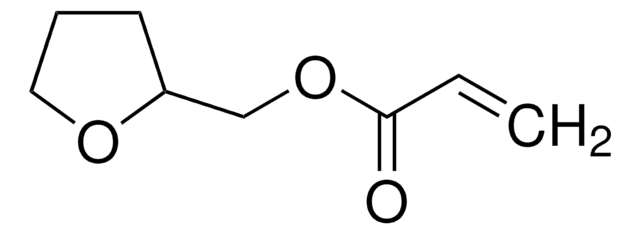
Furfuryl methacrylate
97%, contains 200 ppm monomethyl ether hydroquinone as inhibitor
Synonym(s):
Methacrylic Acid Furfuryl Ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H10O3
CAS Number:
Molecular Weight:
166.17
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
200 ppm monomethyl ether hydroquinone as inhibitor
refractive index
n20/D 1.482 (lit.)
bp
80-82 °C/5 mmHg (lit.)
density
1.078 g/mL at 25 °C (lit.)
SMILES string
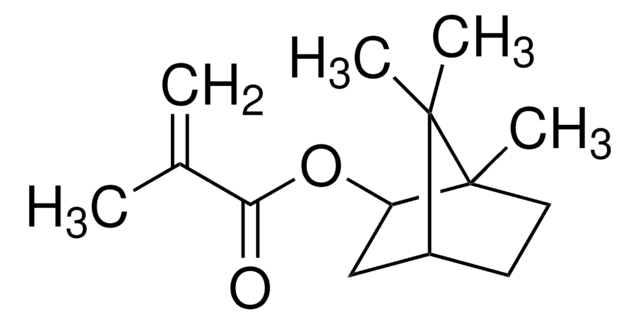
CC(=C)C(=O)OCc1ccco1
InChI
1S/C9H10O3/c1-7(2)9(10)12-6-8-4-3-5-11-8/h3-5H,1,6H2,2H3
InChI key
DWXAVNJYFLGAEF-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sunanda Sain et al.
Polymers, 12(11) (2020-10-30)
The fabrication of smart biocomposites from sustainable resources that could replace today's petroleum-derived polymer materials is a growing field of research. Here, we report preparation of novel biocomposites using nanocellulose networks extracted from food residue (onion skin) and a vegetable
D Zaldívar et al.
Biomaterials, 14(14), 1073-1079 (1993-11-01)
Biocompatible copolymers of N-vinylpyrrolidone (P) and furfuryl methacrylate (F) were prepared by free radical polymerization in N,N-dimethylformamide solution at 50 degrees C, using 2,2'-azobisisobutyronitrile as initiator, at low and high conversion. The microstructure of copolymers prepared at low conversion was
Sunanda Sain et al.
Polymers, 12(2) (2020-01-26)
This work focuses on the development of cross-linked polymer from a highly unsaturated vegetable oil, tung oil (TO) and a bio-based acrylate, furfuryl methacrylate (FMA). The presence of a high degree of unsaturated carbon-carbon bonding in TO makes it a
A Amalin Kavitha et al.
ACS applied materials & interfaces, 1(7), 1427-1436 (2010-04-02)
This investigation reports the effective use of the Diels-Alder (DA) reaction, a "click reaction" in the preparation of thermally amendable and self-healing polymeric materials having reactive furfuryl functionality. In this case, the DA and retro-DA (rDA) reactions were carried out
Sovan Lal Banerjee et al.
Soft matter, 13(47), 9024-9035 (2017-11-28)
Amphiphilic diblock copolymers of poly(furfuryl methacrylate) (PFMA) with cationic poly(2-(methacryloyloxy)ethyltrimethyl ammonium chloride) (PFMA-b-PMTAC) and anionic poly(sodium 4-vinylbenzenesulfonate) (PFMA-b-PSS) were prepared via reversible addition fragmentation chain-transfer (RAFT) polymerization by using PFMA as a macro-RAFT agent. The formation of the block copolymer
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 411760-100ML | 4061837406317 |
| 411760-25ML | 4061837406324 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service