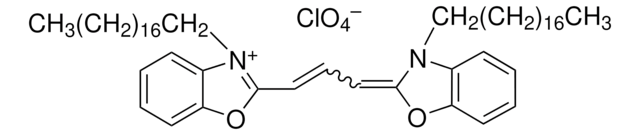
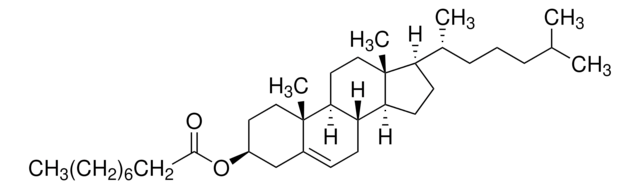
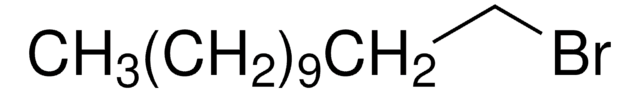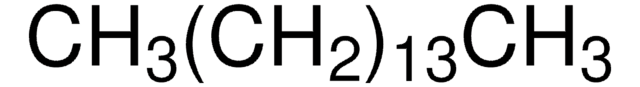410497
4-(Dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran
Dye content 98 %
Synonym(s):
DCM
About This Item
Recommended Products
form
solid
Quality Level
composition
Dye content, 98%
mp
215-220 °C (lit.)
λmax
468 nm
OLED Device Performance
ITO/Alq3:DCM/Alq3/Mg:Ag
ITO/TPD/Alq3:DCM (10%)/Alq3/Mg:Ag
SMILES string
CN(C)c1ccc(\C=C\C2=CC(\C=C(C)O2)=C(\C#N)C#N)cc1
InChI
1S/C19H17N3O/c1-14-10-16(17(12-20)13-21)11-19(23-14)9-6-15-4-7-18(8-5-15)22(2)3/h4-11H,1-3H3/b9-6+
InChI key
YLYPIBBGWLKELC-RMKNXTFCSA-N
Related Categories
General description
Application
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
109.4 °F - closed cup
Flash Point(C)
43 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Developed in the last several years, fluorescence quenching microscopy (FQM) has enabled rapid, inexpensive, and high-fidelity visualization of two-dimensional (2D) materials such as graphene-based sheets and MoS2.
Graphene has emerged as the new wonder material. Being only one atom thick and composed of carbon atoms arranged in a hexagonal honeycomb lattice structure, the interest in this material has exploded exponentially since 2004 when it was first isolated and identified using a very simple method.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 410497-1G | 4061832019543 |
| 410497-250MG | 4061832019550 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)