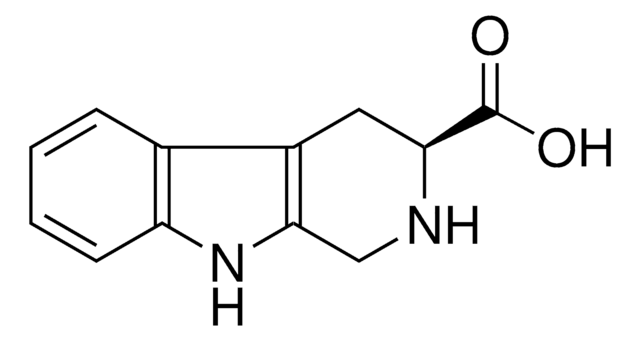
300764
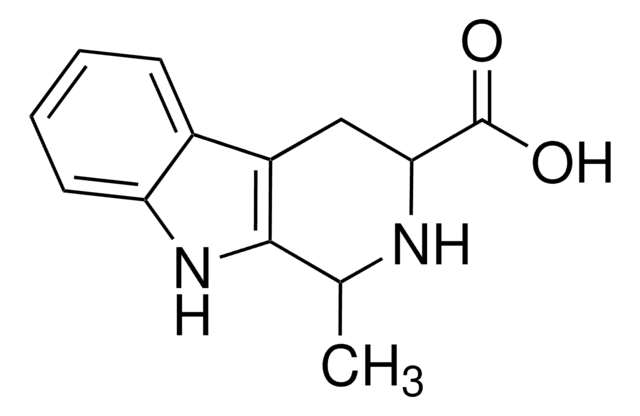
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole
98%
Synonym(s):
Noreleagnine, THBC, Tetrahydronorharman, Tryptoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12N2
CAS Number:
Molecular Weight:
172.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
mp
206-208 °C (lit.)
SMILES string
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
InChI key
CFTOTSJVQRFXOF-UHFFFAOYSA-N
Gene Information
rat ... Htr2a(29595) , Htr2c(25187)
Related Categories
General description
Ozonolysis of the enamine bond of 1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole derivatives was studied.
Application
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole was employed in alkaloid synthesis and in studies on neurodegenerative diseases.
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Karolina Pulka
Current opinion in drug discovery & development, 13(6), 669-684 (2010-11-10)
The synthesis of biologically active heterocyclic scaffolds is one of the significant challenges of modern synthetic chemistry. The Pictet-Spengler (PS) reaction, known for approximately a century, remains a particularly popular cyclization method. This review describes recent applications of the PS
Tetrahedron, 48, 9735-9735 (1992)
Fangrui Wu et al.
Journal of the American Chemical Society, 134(3), 1498-1500 (2012-01-11)
The Pictet-Spenglerase strictosidine synthase (STR1) has been recognized as a key enzyme in the biosynthesis of some 2000 indole alkaloids in plants, some with high therapeutic value. In this study, a novel function of STR1 has been detected which allows
Kaushik Chanda et al.
Molecular diversity, 15(2), 569-581 (2010-10-12)
The Pictet-Spengler reaction, using polyethylene glycol immobilized tryptophan ester with a variety of ketones, was achieved by refluxing condition in acidic chloroform. The linear as well as cyclic ketones were employed. All the ketones were reacted within 6-8 h to
I M McDonald et al.
Journal of medicinal chemistry, 43(19), 3518-3529 (2000-09-23)
A novel series of nonpeptide CCK(2) receptor antagonists has been prepared, in which 2,7-dioxo-2,3,4,5,6,7-hexahydro-1H-benzo[h][1, 4]diazonine (5) was used as a chemical template. This uncommon ring system was obtained in a highly substituted form and in high yield by ozonolysis of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 300764-1G | 4061826659427 |
| 300764-5G | 4061832841625 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
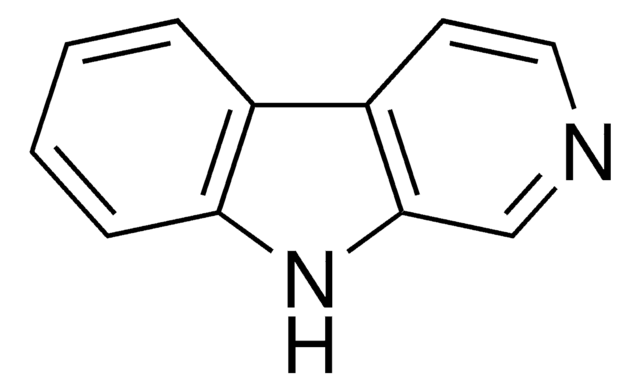
![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)