All Photos(1)
About This Item
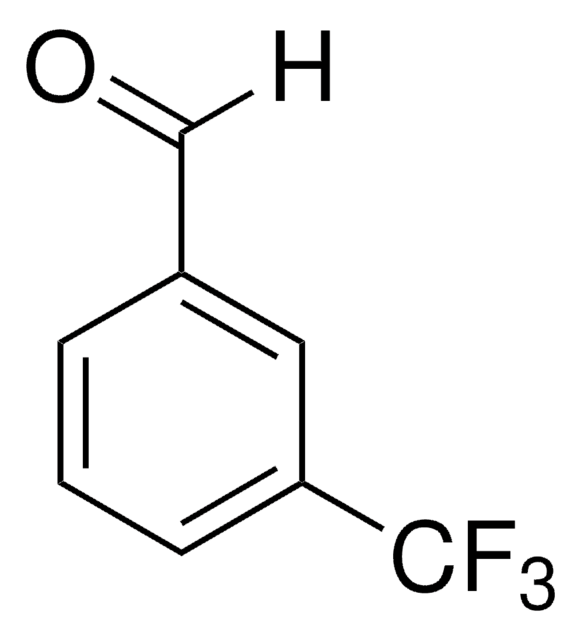
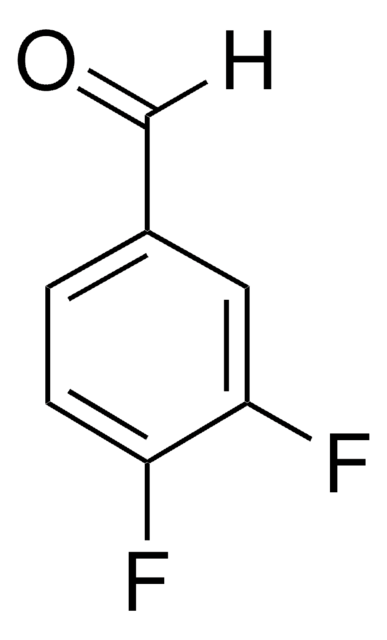
Linear Formula:
(CF3)2C6H3CHO
CAS Number:
Molecular Weight:
242.12
Beilstein:
1884058
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.422 (lit.)
bp
37 °C/1.3 mmHg (lit.)
density
1.469 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI
1S/C9H4F6O/c10-8(11,12)6-1-5(4-16)2-7(3-6)9(13,14)15/h1-4H
InChI key
LDWLIXZSDPXYDR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Bis(trifluoromethyl)benzaldehyde was used in the synthesis of of a series of meso-3,5-bis(trifluoromethyl)phenyl-substituted expanded porphyrins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Soonchul Kang et al.
Chemistry, an Asian journal, 3(12), 2065-2074 (2008-11-14)
Trifluoroacetic acid-catalyzed condensation of pyrrole with electron-deficient and sterically hindered 3,5-bis(trifluoromethyl)benzaldehyde results in the unexpected production of a series of meso-3,5-bis(trifluoromethyl)phenyl-substituted expanded porphyrins including [22]sapphyrin 2, N-fused [22]pentaphyrin 3, [26]hexaphyrin 4, and intact [32]heptaphyrin 5 together with the conventional 5,10,15,20-tetrakis(3,5-bis(trifluoromethyl)phenyl)porphyrin
M M V Ramana et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 152, 165-171 (2015-07-25)
In the present work, isopropyl-6-amino-4-(3,5-bis(trifluoromethyl)phenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate (4H-pyran analog) has been synthesized by a three component reaction catalyzed by CsOH/γ-Al2O3 and characterized. The interaction of 4H-pyran analog with herring sperm DNA (hs DNA) under physiological conditions (phosphate buffer of pH 7.2) was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service