All Photos(3)
About This Item
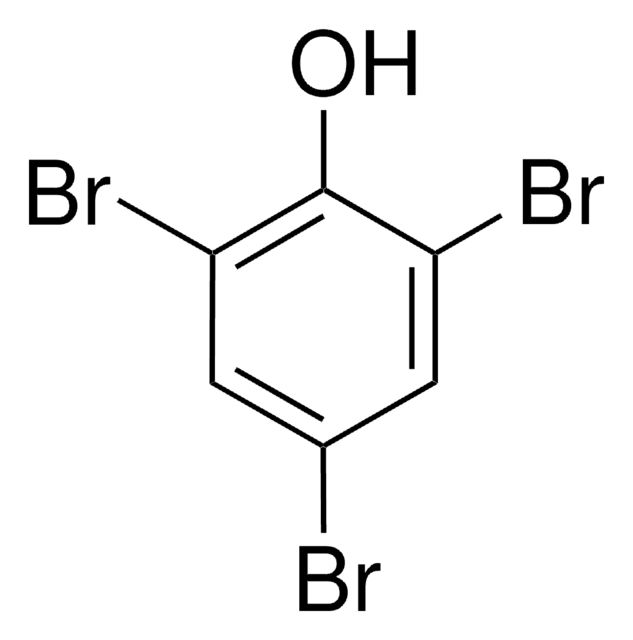
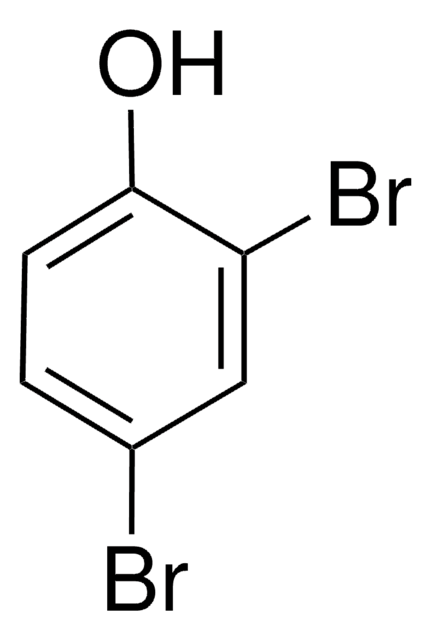
Linear Formula:
Br2C6H3OH
CAS Number:
Molecular Weight:
251.90
Beilstein:
2043614
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
255-256 °C/740 mmHg (lit.)
mp
53-56 °C (lit.)
functional group
bromo
SMILES string
Oc1c(Br)cccc1Br
InChI
1S/C6H4Br2O/c7-4-2-1-3-5(8)6(4)9/h1-3,9H
InChI key
SSIZLKDLDKIHEV-UHFFFAOYSA-N
Gene Information
human ... ALOX12(239) , ALOX15(246) , GABRA1(2554)
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Dibromophenol has been isolated from a luminous marine enteropneust, Balanoglossus biminiensis and is responsible for the characteristic ″iodoform-like″ odor of these animals.
Application
2,6-Dibromophenol has been used in the preparation of 2-(2,6-dibromophenoxy)ethylbromide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Caroline Vaaland et al.
Chemosphere, 256, 126928-126928 (2020-05-23)
Phenols and trans-1,2-dihydro-1,2-diols are metabolites commonly formed in vivo in fish upon exposure to polycyclic aromatic hydrocarbons (PAHs). These metabolites are excreted via the bile and gas chromatography-mass spectrometry (GC-MS) analysis of bile is becoming more frequently used for evaluating PAH
Synthesis of arylpiperazines via palladium-catalysed aromatic amination reactions of bromoarenes with N- tert-butoxycarbonylpiperazine.
Kerrigan F, et al.
Tetrahedron Letters, 39(15), 2219-2222 (1998)
Satoko Shigetatsu et al.
Journal of environmental science and health. Part A, Toxic/hazardous substances & environmental engineering, 45(12), 1536-1542 (2010-08-20)
An iron(III)-porphyrin catalyst, iron(III)-tetrakis(p-hydroxyphenyl)porphyrin (FeTHP), was introduced into a humic acid via a formaldehyde or urea-formaldehyde polycondensation reaction to stabilize the catalyst. The prepared supramolecular catalysts were then attached to Dowex-22, an anion-exchange resin. The oxidation of 2,6-dibromophenol (2,6-DBP) was
J F Charles
Annales de microbiologie, 134A(3), 365-381 (1983-05-01)
The effects of Bacillus thuringiensis var. israelensis delta-endotoxin were investigated on a cell culture of Aedes aegypti with the electron microscope. The ultrastructural changes following intoxication were: disintegration of endoplasmic reticula by the formation of spherical structures; condensation and then
Noel Ferro et al.
Phytochemistry, 68(2), 237-250 (2006-11-28)
An computational-biostatistical approach, supported by ab initio optimizations of auxin-like molecules, was used to find biologically meaningful relationships between quantum chemical variables and fresh bioassay's data. It is proven that the auxin-like recognition requires different molecular assembling states. We suggest
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service