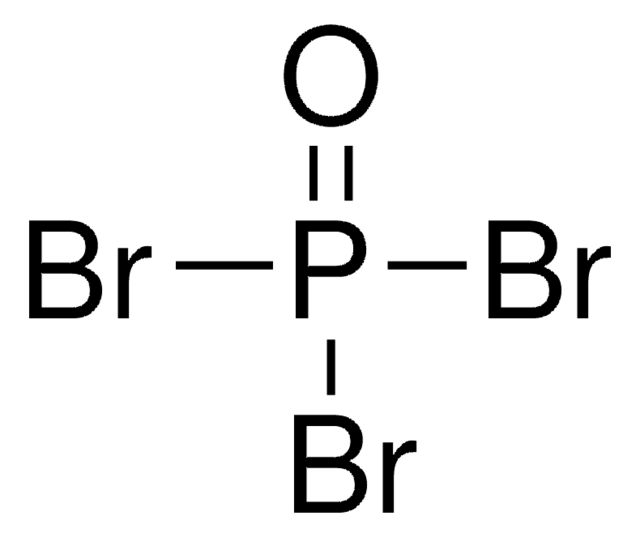251259
Thionyl bromide
97%
Synonym(s):
Dibromo sulfoxide, Sulfur bromide oxide (SOBr2)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
SOBr2
CAS Number:
Molecular Weight:
207.87
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
10 mmHg ( 31 °C)
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.675 (lit.)
bp
48 °C/20 mmHg (lit.)
mp
−52 °C (lit.)
density
2.683 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
BrS(Br)=O
InChI
1S/Br2OS/c1-4(2)3
InChI key
HFRXJVQOXRXOPP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Thionyl bromide (SOBr2) can be used as a brominating agent in organic synthesis for the bromination of alcohols, carboxylic acids, alkenes, and ketones.
SoBr2 can be used in the synthesis of:
SoBr2 can be used in the synthesis of:
- ortho
- -Bromoanilides by bromination of ortho C-H bonds of the aryhydroxylamines.
- β-Amino bromides from β-aminoalcohols in the presence of DMF as a solvent.
- 4,8-Dibromobenzo[1,2-c;4,5-c′]bis[1,2,5]thiadiazole from 1,2,4,5-tetraaminobenzene tetrahydrobromide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient synthesis of beta-amino bromides
Nagle AS, et al.
Tetrahedron Letters, 41(17), 3011-3014 (2000)
Practical bromination of arylhydroxylamines with SOBr2 towards ortho-bromo-anilides
Du Yuanbo, et al.
Tetrahedron Letters, 72, 153074-153074 (2021)
Palladium-catalyzed alpha-arylation of aldehydes with bromo- and chloroarenes catalyzed by [{Pd(allyl)Cl}2] and dppf or Q-phos.
Giang D Vo et al.
Angewandte Chemie (International ed. in English), 47(11), 2127-2130 (2008-02-09)
Hiroyuki Nakamura et al.
Organic letters, 5(8), 1167-1169 (2003-04-12)
[reaction: see text] A perfluorohexane layer regulates the rate of reagent transport in the bromination and chlorination of alcohols. A fluorous triphasic U-tube method is effective for lighter reagents; the thionyl chloride layer (yellow) vanishes, and the chlorides are obtained
Thionyl bromide
Ho TL, et al.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service