All Photos(2)
About This Item
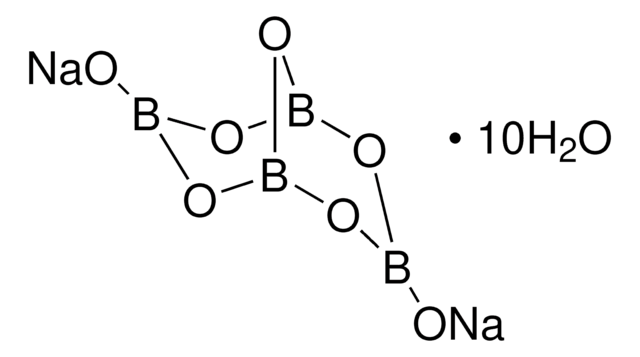
Linear Formula:
Na2B4O7
CAS Number:
Molecular Weight:
201.22
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Assay:
99%
form:
solid
Recommended Products
Agency
suitable for SM 4500 - NH3
Quality Level
Assay
99%
form
solid
mp
741 °C (lit.)
density
2.367 g/mL at 25 °C (lit.)
SMILES string
[Na+].[Na+].[O-]B1Ob2ob([O-])ob(O1)o2
InChI
1S/B4O7.2Na/c5-1-7-3-9-2(6)10-4(8-1)11-3;;/q-2;2*+1
InChI key
UQGFMSUEHSUPRD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Sodium tetraborate (Na2B4O7) can be used as a co-catalyst for the oxidation of alcohols to corresponding carbonyl compounds in greener non-chlorinated solvents in the presence of TEMPO/NaOCl. It is also used as a structure-directing agent as well as a catalyst in the preparation of carbon aerogels using glucose as the carbon precursor.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Repr. 1B
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Borax-Mediated Formation of Carbon Aerogels from Glucose
Fellinger T-P, et al.
Advances in Functional Materials, 22(15), 3254-3260 (2012)
Zichen Zhao et al.
Foods (Basel, Switzerland), 10(7) (2021-08-08)
UV-B illumination facilitates aggregation of alpha-lactalbumin (α-LA) by intramolecular disulfide bond cleavage followed by intermolecular thiol-disulfide exchange reactions. However, long term exposure to UV-B illumination may induce undesired oxidative modifications of amino acid residues in the protein. The purpose of
Jamshed Iqbal et al.
Journal of chromatography. A, 1218(29), 4764-4771 (2011-06-15)
A simple, efficient, and highly sensitive in-line CE method was developed for the characterization and for inhibition studies of the nucleoside-metabolizing enzymes purine nucleoside phosphorylase (PNP) and adenosine deaminase (ADA) present in membrane preparations of human 1539 melanoma cells. After
Ruina Li et al.
Journal of hazardous materials, 248-249, 268-275 (2013-02-07)
Rapid analysis of trace amount of aromatic amines in environmental samples and daily necessities has attracted considerable attentions because some of them are strongly toxic and carcinogenic. In this study, fast and efficient electrophoretic separation and sensitive determination of 5
Tingyao Zhou et al.
Nanoscale, 4(17), 5312-5315 (2012-07-28)
A facile approach was developed to prepare positively charged and red-emitting lysozyme-stabilized Ag nanoclusters (Lys-AgNCs) using NaBH₄ as a reducing agent at room temperature. The Lys-AgNCs can be applied in the highly selective detection of Hg²⁺.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service