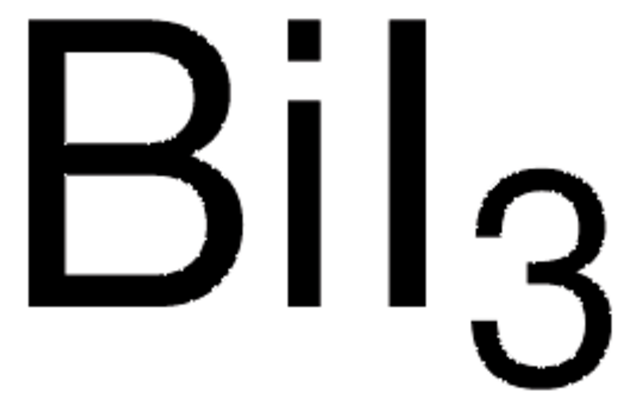202134
Cesium iodide
99.9% trace metals basis
Synonym(s):
Caesium iodide, Caesium monoiodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
CsI
CAS Number:
Molecular Weight:
259.81
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Assay
99.9% trace metals basis
form
powder
impurities
≤1500.0 ppm Trace Metal Analysis
mp
626 °C (lit.)
density
4.51 g/mL at 25 °C (lit.)
SMILES string
[I-].[Cs+]
InChI
1S/Cs.HI/h;1H/q+1;/p-1
InChI key
XQPRBTXUXXVTKB-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Cesium iodide (CsI) can be used as a cathode for the fabrication of field emission cathodes for potential usage in high power microwave systems. It can also be used to form an interface layer between the blocking layer and the perovskite active layer to improve the photovoltaic performance and stability of solar cells.
Features and Benefits
Frequently used in devices such as phosphor screens for medical imaging, scintillators, calorimeters and a variety of particle detectors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Repr. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Low level plasma formation in a carbon velvet cesium iodide coated cathode
Shiffler D, et al.
Physics of Plasmas, 11(4), 1680-1684 (2004)
Comparison of velvet-and cesium iodide-coated carbon fiber cathodes
Shiffler D, et al.
IEEE transactions on nuclear science, 29(3), 445-451 (2001)
Olga Nazarenko et al.
Inorganic chemistry, 56(19), 11552-11564 (2017-09-13)
Interest in hybrid organic-inorganic lead halide compounds with perovskite-like two-dimensional crystal structures is growing due to the unique electronic and optoelectronic properties of these compounds. Herein, we demonstrate the synthesis, thermal and optical properties, and calculations of the electronic band
Cong Chen et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 6(11), 1802046-1802046 (2019-06-11)
Photovoltaic devices employing lead halide perovskites as the photoactive layer have attracted enormous attention due to their commercialization potential. Yet, there exists challenges on the way to the practical use of perovskite solar cells (PSCs), such as light stability and
Taehwan Jun et al.
Advanced materials (Deerfield Beach, Fla.), 30(43), e1804547-e1804547 (2018-09-15)
Halide perovskites, including CsPbX3 (X = Cl, Br, I), have gained much attention in the field of optoelectronics. However, the toxicity of Pb and the low photoluminescence quantum yield (PLQY) of these perovskites hamper their use. In this work, new
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service