197637
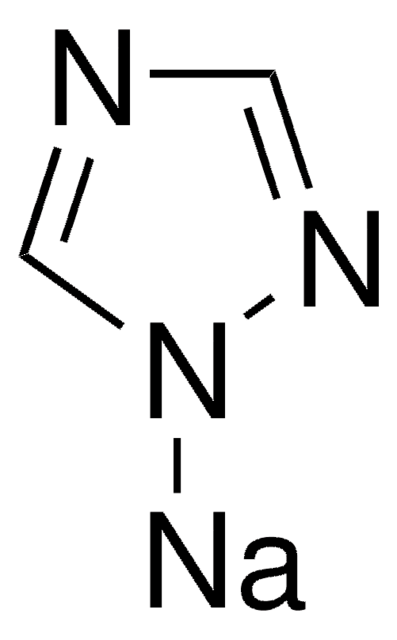
Imidazole sodium derivative
technical grade
Synonym(s):
Imidazolylsodium, Sodium imidazolide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H3N2Na
CAS Number:
Molecular Weight:
90.06
Beilstein:
3569312
EC Number:
MDL number:
UNSPSC Code:
12352005
eCl@ss:
39161001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
mp
284 °C (dec.) (lit.)
SMILES string
[Na]n1ccnc1
InChI
1S/C3H3N2.Na/c1-2-5-3-4-1;/h1-3H;/q-1;+1
InChI key
ITAWMPSVROAMOE-UHFFFAOYSA-N
Application
Imidazole sodium derivative (Imidazolylsodium) was used in the synthesis of arylazidoamorphigenin.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F G Earley et al.
The Biochemical journal, 224(2), 525-534 (1984-12-01)
A photoaffinity-labelling analogue of the respiratory inhibitor rotenone was synthesized from the naturally occurring rotenoid amorphigenin. The analogue inhibits NADH-ubiquinone oxidoreductase activity at concentrations comparable with those of rotenone. Photolysis of the radiolabelled analogue bound to isolated NADH-ubiquinone oxidoreductase resulted
Richard C Knighton et al.
Chemical communications (Cambridge, England), 49(23), 2293-2295 (2013-02-15)
The dansyl fluorophore ligated to gold nanoparticles via imidazole and amine groups affords conjugates capable of detecting micromolar concentrations of the chemical warfare agent sulfur mustard by a fluorescence switching 'ON' displacement assay.
Yin Gao et al.
Bioorganic & medicinal chemistry, 21(5), 1305-1311 (2013-02-05)
Galactosyltransferases (GalTs) extend the glycan chains of mammalian glycoproteins by adding Gal to terminal GlcNAc residues, and thus build the scaffolds for biologically important glycan structures. We have shown that positively charged bivalent imidazolium salts in which the two imidazolium
Chuanjiang Hu et al.
Inorganic chemistry, 52(6), 3170-3177 (2013-03-09)
The effects of the deprotonation of coordinated imidazole on the vibrational dynamics of five-coordinate high-spin iron(II) porphyrinates have been investigated using nuclear resonance vibrational spectroscopy. Two complexes have been studied in detail with both powder and oriented single-crystal measurements. Changes
Ho Young Lee et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(14), 5416-5421 (2013-03-16)
RNA-binding proteins control the fate and function of the transcriptome in all cells. Here we present technology for isolating RNA-protein partners efficiently and accurately using an engineered clustered regularly interspaced short palindromic repeats (CRISPR) endoribonuclease. An inactive version of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service