All Photos(1)
About This Item
Empirical Formula (Hill Notation):
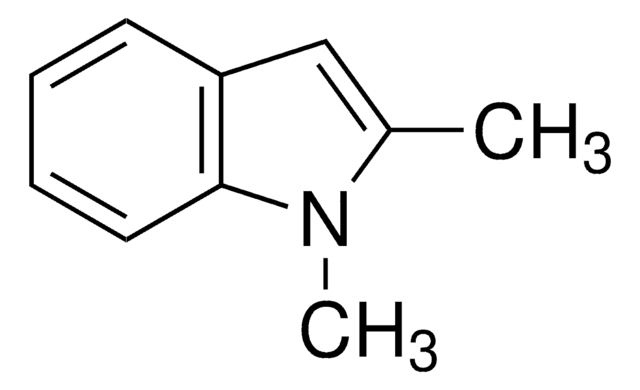
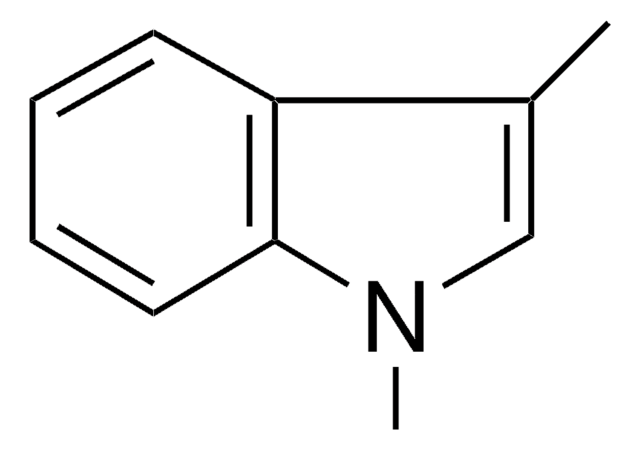
C9H9N
CAS Number:
Molecular Weight:
131.17
Beilstein:
111026
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
liquid
refractive index
n20/D 1.606 (lit.)
bp
133 °C/26 mmHg (lit.)
density
1.051 g/mL at 20 °C (lit.)
SMILES string
Cn1ccc2ccccc12
InChI
1S/C9H9N/c1-10-7-6-8-4-2-3-5-9(8)10/h2-7H,1H3
InChI key
BLRHMMGNCXNXJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Methylindole undergoes Au(III)/TPPMS-catalyzed benzylation reaction with benzhydryl and benzylic alcohols.
Application
1-Methylindole was used in the determination of association constant for the electron-donor-acceptor complexes of 1-methylindole with 1-(2,4,6-trinitrophenyl) propan-2-one.
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Non-receptor tyrosine kinase (Src kinase) inhibitors
- PET agents for imaging of protein kinase C (PKC)
- Ynediones as highly reactive Michael systems
- Anticancer agents
- Polycyclic derivatives of indoles
- PET agents for imaging of glycogen synthase kinase-3 (GSK-3)
- Anti-prion disease agents
- Bisindole derivatives with antihyperlipidemic activity
- PET cancer imaging agents
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hidemasa Hikawa et al.
The Journal of organic chemistry, 78(23), 12128-12135 (2013-11-22)
A novel and efficient method for the Au(III)/TPPMS-catalyzed direct substitution reaction of benzhydryl and benzylic alcohols with indoles in water is developed. Au(III)/TPPMS is an effective catalyst for the benzylation of the strong π nucleophile 1-methylindole, while common Brønsted or
Martin G Banwell et al.
Organic letters, 8(21), 4959-4961 (2006-10-06)
[reaction: see text] Reaction of N-methylindole (4) with 6,6-dibromobicyclo[3.1.0]hexane (5) in the presence of silver tetrafluoroborate affords conjugate 7 in 67% yield. This product can be readily elaborated to compounds 12b and 13b which embody the polycyclic frameworks associated with
Hongmei Liu et al.
Malaria journal, 17(1), 348-348 (2018-10-07)
Anopheles sinensis is an important vector for the spread of malaria in China. Olfactory-related behaviours, particularly oviposition site seeking, offer opportunities for disrupting the disease-transmission process. This is the first report of the identification and characterization of AsinOrco and AsinOR10
Association constants for the electron-donor-acceptor complexes of indole and 1-methylindole with 1-(2, 4, 6-trinitrophenyl) propan-2-one from nuclear magnetic resonance shift measurements. An anomalous scatchard plot.
Chudek JA, et al.
J. Chem. Soc., Faraday, 84(4), 1145-1152 (1988)
Nicholas R Deprez et al.
Journal of the American Chemical Society, 128(15), 4972-4973 (2006-04-13)
This communication describes the rational development of a PdII-catalyzed method for the direct 2-arylation of indoles using [Ar-IIII-Ar]BF4. These reactions proceed under remarkably mild conditions (often at room temperature and in the presence of ambient air and moisture), and these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service