182702
Poly(vinylidene fluoride)
average Mw ~534,000 by GPC, powder
Synonym(s):
PVDF
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
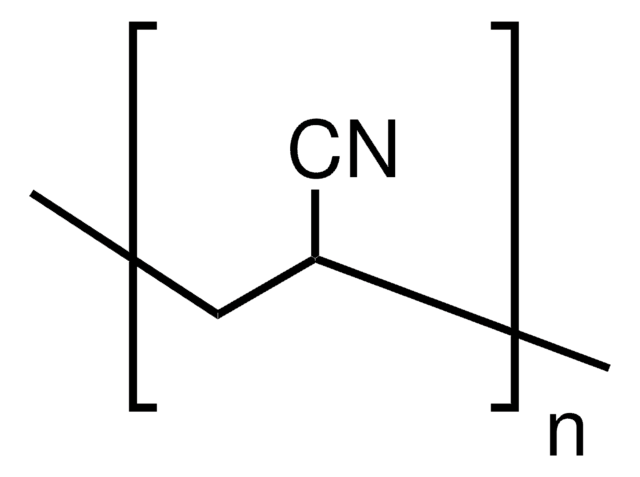
Linear Formula:
(CH2CF2)n
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
15 mmHg ( 32 °C)
form
powder
mol wt
average Mw ~534,000 by GPC
refractive index
n20/D 1.42
transition temp
Tg −38 °C
Tm 171 °C
density
1.74 g/mL at 25 °C
SMILES string
FC(F)=C
InChI
1S/C2H2F2/c1-2(3)4/h1H2
InChI key
BQCIDUSAKPWEOX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Poly(vinylidene fluoride) PVDF is a semi crystalline non-centrosymmetric polymer which exhibits piezo-, pyro- and ferroelectric properties. It is a linear polymer that shows permanent electric dipoles perpendicular to the direction of the molecular chain. These dipoles result from the difference in electronegativity between the atoms of hydrogen and fluorine with respect to carbon. Depending on the processing conditions, PVDF exhibits several different crystalline phases (α,β,γ,δ). The β phase of PVDF is the phase that exhibits the best ferroelectric and piezoelectric properties.
Application
Poly(vinylidene fluoride) (PVDF) is used in nanoporous electrodes. It is used to synthesize composites comprising of ultrafine fibers of PVDF embedded with carbon nanotubes. It can be used in sensors, actuators, for energy storage, sphygmomanometers, pressure sensors and nanogenerators, lithium ion batteries. Additionally, it may find application as matrix for PVDF-nanoclay nanocomposites and PVDF acrylic rubber/clay nanocomposite hybrid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohammad Mahdi Abolhasani et al.
PloS one, 9(2), e88715-e88715 (2014-02-20)
In this paper, intercalation of nanoclay in the miscible polymer blend of poly(vinylidene fluoride) (PVDF) and acrylic rubber(ACM) was studied. X-ray diffraction was used to investigate the formation of nanoscale polymer blend/clay hybrid. Infrared spectroscopy and X-ray analysis revealed the
Hot Plate Annealing at a Low Temperature of a Thin Ferroelectric P(VDF-TrFE) Film with an Improved Crystalline Structure for Sensors and Actuators.
Sensors (Basel, Switzerland), 14(10), 19115-19127 null
Chi Yan Lai et al.
Membranes, 4(1), 55-78 (2014-06-25)
Current commercial polymer membranes have shown high performance and durability in water treatment, converting poor quality waters to higher quality suitable for drinking, agriculture and recycling. However, to extend the treatment into more challenging water sources containing abrasive particles, micro
Wenzhong Ma et al.
Membranes, 4(2), 243-256 (2014-06-25)
The crystallization behaviors of two copolymers of PVDF were studied, and the effect of copolymerized chains on the crystallization behavior was investigated. The results indicated that both copolymers had a lowered crystallization temperature and crystallinity. The crystallization rate was improved
Thermal-to-electric energy conversion of a nanoporous carbon
Yu Q, et al
Journal of Power Sources, 183(1), 403-405 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service