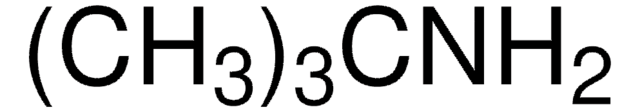176990
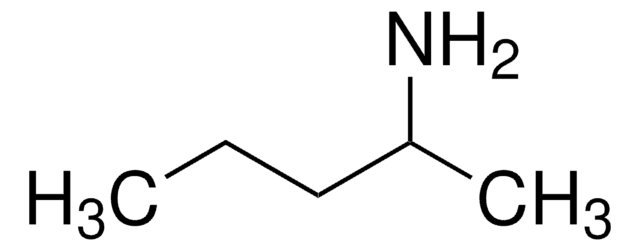
tert-Amylamine
98%
Synonym(s):
1,1-Dimethylpropylamine, tert-Pentylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5C(CH3)2NH2
CAS Number:
Molecular Weight:
87.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.3996 (lit.)
bp
77 °C (lit.)
density
0.746 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCC(C)(C)N
InChI
1S/C5H13N/c1-4-5(2,3)6/h4,6H2,1-3H3
InChI key
GELMWIVBBPAMIO-UHFFFAOYSA-N
Related Categories
Application
tert-Amylamine was used as a substitute for t-butylamino substituent in cyanobenzylamine derivative.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Afrooz Shiekhzadeh et al.
Applied biochemistry and biotechnology, 190(2), 506-528 (2019-08-08)
In this paper, a new anticancer Pt (II) complex, cis-[Pt (NH3)2(tertpentylgly)]NO3, was synthesized with glycine-derivative ligand and characterized. Cytotoxicity of this water-soluble Pt complex was studied against human cancer breast cell line of MCF-7. The interaction of human serum albumin
A M Gilbert et al.
Journal of medicinal chemistry, 43(6), 1203-1214 (2000-03-29)
A novel series of benzylamine, potassium channel openers (KCOs) is presented as part of our program toward designing new, bladder-selective compounds for the treatment of urge urinary incontinence (UUI). We have found that the in vitro potency of (R)-4-[3,4-dioxo-2-(1,2, 2-trimethyl-propylamino)-cyclobut-1-enylamino]-3-ethyl-benzo
Mahsa Zahiri et al.
Journal of cellular physiology, 235(2), 1036-1050 (2019-07-06)
In this study, the chemical features of dendritic mesoporous silica nanoparticles (DMSNs) provided the opportunity to design a nanostructure with the capability to intelligently transport the payload to the tumor cells. In this regard, doxorubicin (DOX)-encapsulated DMSNs was electrostatically surface-coated
Radhe Shyam et al.
Biopolymers, 110(6), e23273-e23273 (2019-03-22)
The design of linear peptoid oligomers adopting well-defined secondary structures while mimicking defined peptide primary sequences is a major challenge in the context of drug discovery. To this end, chemists have developed cis-inducing peptoid side chains to build robust polyproline
Jan Leipert et al.
Analytical and bioanalytical chemistry, 410(19), 4737-4748 (2018-02-23)
The identification and quantification of molecules involved in bacterial communication are major prerequisites for the understanding of interspecies interactions at the molecular level. We developed a procedure allowing the determination of 2-heptyl-4(1H)-quinolone (HHQ) and 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS) and the virulence factor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service