151653
Methyl hydrazinocarboxylate
97%
Synonym(s):
Methoxycarbonylhydrazine, Methyl carbazate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NNHCO2CH3
CAS Number:
Molecular Weight:
90.08
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
108 °C/12 mmHg (lit.)
mp
70-73 °C (lit.)
SMILES string
COC(=O)NN
InChI
1S/C2H6N2O2/c1-6-2(5)4-3/h3H2,1H3,(H,4,5)
InChI key
WFJRIDQGVSJLLH-UHFFFAOYSA-N
Application
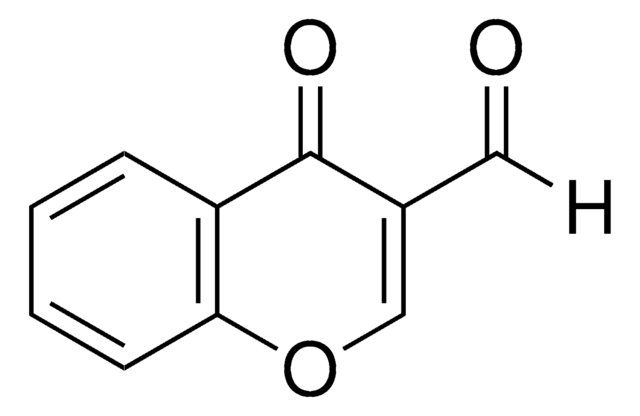
Methyl hydrazinocarboxylate (Methyl carbazate) was used in the preparation of methyl 3-(4-methyl-benzyl-idene)carbazate. It was also used in the synthesis of imidazo[1,5-d][1,2,4]triazines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu-Feng Li et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 6), o1429-o1429 (2010-01-01)
The title compound, C(10)H(12)N(2)O(2), was prepared by the reaction of methyl carbazate and 4-methyl-benzaldehyde. The dihedral angle between the benzene ring and the carbazate fragment is 20.86 (10)°. In the crystal structure, mol-ecules are linked by inter-molecular N-H⋯O hydrogen bonds.
R Truhaut et al.
Toxicology, 22(3), 219-221 (1981-01-01)
Methyl carbazate, a metabolite of carbadox in the rat, was administered orally to rats for 2 years. The compound was mixed in the diet at concentrations corresponding to dose levels of 0, 2.5, 5 and 10 mg/kg body wt/day. There
R Paul et al.
Journal of medicinal chemistry, 28(11), 1704-1716 (1985-11-01)
By using inhibition of histamine release from antigen-challenged, sensitized human basophils as a means of identifying a potentially prophylactic drug for the treatment of asthma, a series of substituted imidazo[1,5-d][1,2,4]triazines were found, which were active. These compounds were prepared by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service