All Photos(2)
About This Item
Empirical Formula (Hill Notation):
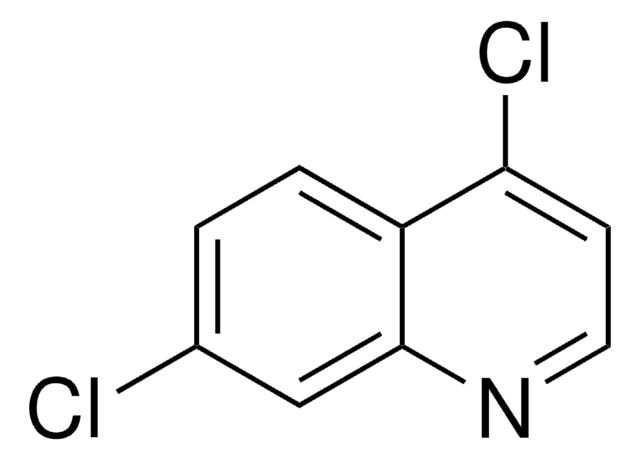
C9H5Cl2N
CAS Number:
Molecular Weight:
198.05
Beilstein:
125359
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
solid
mp
81-83 °C (lit.)
solubility
chloroform: soluble 50 mg/mL, clear, colorless to greenish-yellow
functional group
chloro
SMILES string
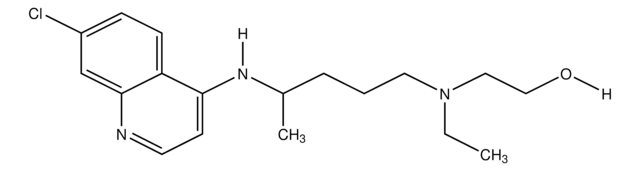
Clc1ccc2c(Cl)ccnc2c1
InChI
1S/C9H5Cl2N/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H
InChI key
HXEWMTXDBOQQKO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4,7-Dichloroquinoline was used in the synthesis of piperaquine. It was used as starting reagent in the synthesis of {3-amino-5-[(7-chloro-4-quinolyl)amino]phenyl}methanol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Lindegårdh et al.
Journal of chromatography. A, 1135(2), 166-169 (2006-10-19)
A significant contaminant of the antimalarial drug piperaquine (1,3-bis-[4-(7-chloroquinolyl-4)-piperazinyl-1]propane) has been identified using liquid chromatography-mass spectrometry (LC-MS) and 2D NMR spectroscopy (1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC). The impurity was identified as the positional isomer 1-[(5-chloroquinolin-4)-piperazinyl]-3-[(7-chloroquinolin-4)-piperazinyl]propane. The impurity is formed
S Delarue et al.
Chemical & pharmaceutical bulletin, 49(8), 933-937 (2001-08-23)
Amodiaquine (AQ) is an antimalarial which is effective against chloroquino-resistant strains of Plasmodium falciparum but whose clinical use is severely restricted because of associated hepatotoxicity and agranulocytosis. "One-pot" synthesis of formamidines likely to be transformed into AQ derivatives is reported.
Elaine S Coimbra et al.
Chemical biology & drug design, 75(6), 628-631 (2010-03-27)
We report herein the condensation of 4,7-dichloroquinoline (1) with tryptamine (2) and D-tryptophan methyl ester (3). Hydrolysis of the methyl ester adduct (5) yielded the free acid (6). The compounds were evaluated in vitro for activity against four different species
J T Mague et al.
Acta crystallographica. Section C, Crystal structure communications, 51 ( Pt 7), 1423-1425 (1995-07-15)
The title compound C14H12C12N2O, has been shown to have an E configuration about the double bond in the propenal moiety. Significant delocalization of the lone pair on the N atom of the dimethylamino group into the pi system of this
Allergic contact dermatitis from 4,7-dichloroquinoline.
F C Pickering et al.
Contact dermatitis, 8(4), 269-270 (1982-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service