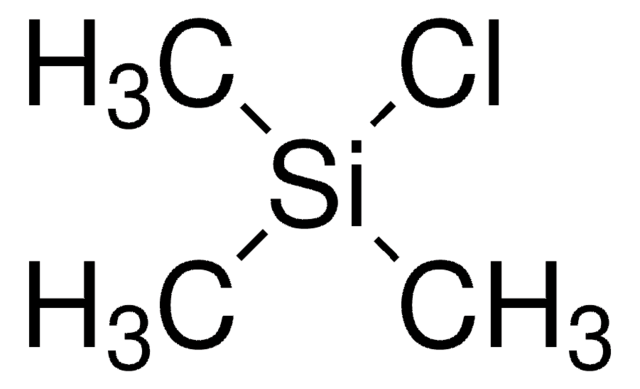125512
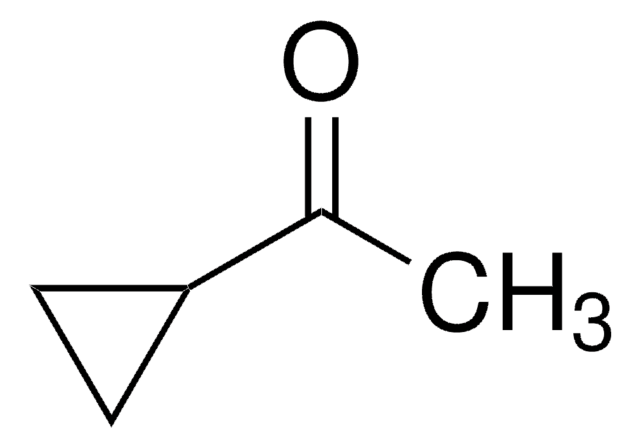
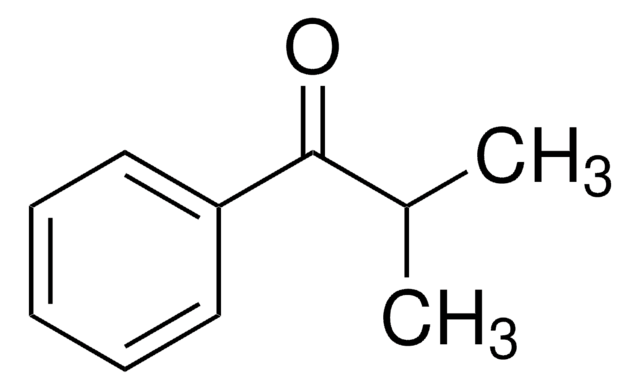
Cyclopropyl phenyl ketone
97%
Synonym(s):
Benzoylcyclopropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COC3H5
CAS Number:
Molecular Weight:
146.19
Beilstein:
1860145
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5 (vs air)
Quality Level
Assay
97%
refractive index
n20/D 1.553 (lit.)
bp
121-123 °C/15 mmHg (lit.)
mp
7-9 °C (lit.)
density
1.058 g/mL at 25 °C (lit.)
SMILES string
O=C(C1CC1)c2ccccc2
InChI
1S/C10H10O/c11-10(9-6-7-9)8-4-2-1-3-5-8/h1-5,9H,6-7H2
InChI key
PJRHFTYXYCVOSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
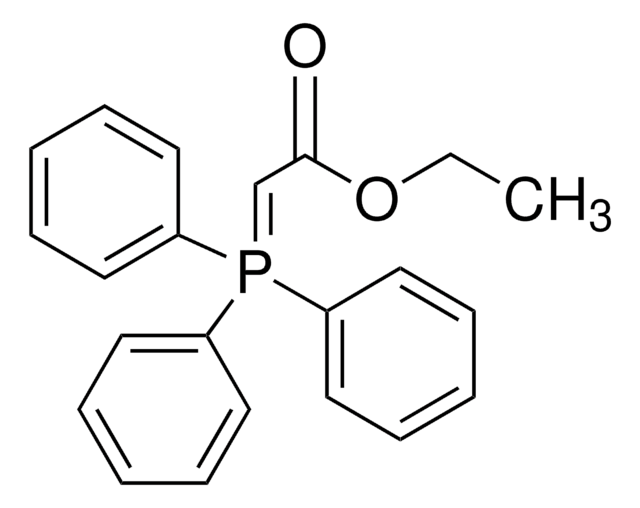
Cyclopropyl phenyl ketone was used to prepare α-cyclopropylstyrene by the Wittig reaction in dimethyl sulfoxide. It was used as starting reagent during the (Z)-titanium enolate formation from cyclopropyl ketones via TiCl4-n-Bu4NI-induced ring opening reaction.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclopropyl substituent effects on acid-catalyzed hydration of alkenes. Correlation by σ+ parameters.
Oyama K and Tidwell TT.
Journal of the American Chemical Society, 98(4), 947-951 (1976)
Enolate formation from cyclopropyl ketones via iodide-induced ring opening and its use for stereoselective aldol reaction.
Han Z, et al.
Tetrahedron, 57(6), 987-995 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service