1711009
USP
Vainillina
United States Pharmacopeia (USP) Reference Standard
Sinónimos:
4-Hidroxi-3-metoxibenzaldehído, Aldehído vanílico
About This Item
Productos recomendados
grade
pharmaceutical primary standard
vapor density
5.3 (vs air)
vapor pressure
>0.01 mmHg ( 25 °C)
API family
vanillin
manufacturer/tradename
USP
bp
170 °C/15 mmHg (lit.)
mp
81-83 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
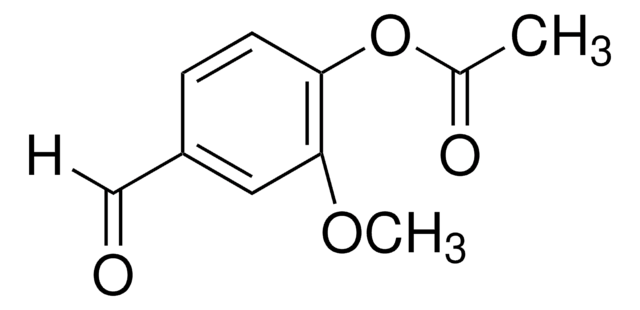
COc1cc(C=O)ccc1O
InChI
1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3
InChI key
MWOOGOJBHIARFG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
It has been used as reference standard in calibrating hot stage; during thermomicroscopic investigation performed with optical polarizing microscope; usually equipped with a hot stage and temperature controller.
Analysis Note
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
319.6 - 321.4 °F - closed cup
flash_point_c
159.8 - 160.8 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico