T9825
Tau-383 human
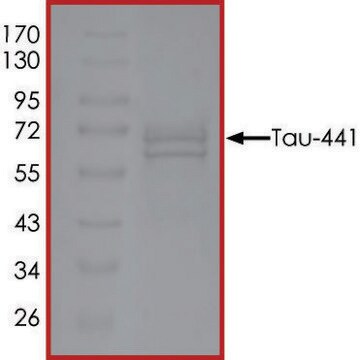
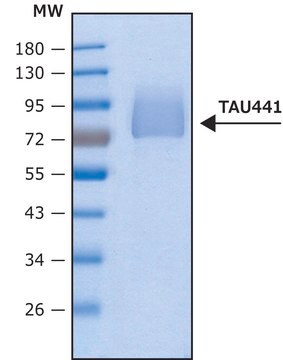
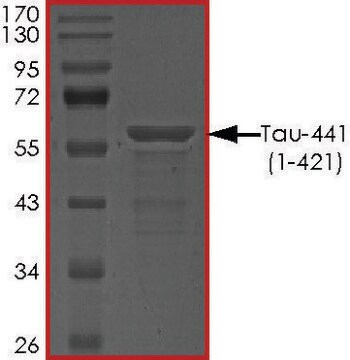
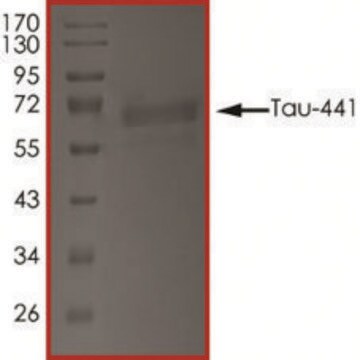
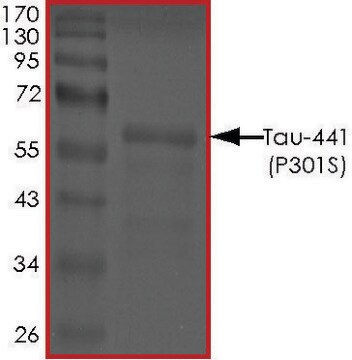
recombinant, expressed in E. coli, ≥90% (SDS-PAGE), lyophilized powder
Sinónimos:
MAPTL, MTBT1, TAU
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
biological source
human
Quality Level
recombinant
expressed in E. coli
assay
≥90% (SDS-PAGE)
form
lyophilized powder
mol wt
40 kDa
technique(s)
western blot: suitable
suitability
suitable for Western blot
UniProt accession no.
application(s)
cell analysis
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... MAPT(4137)
General description
Research area: Neuroscience
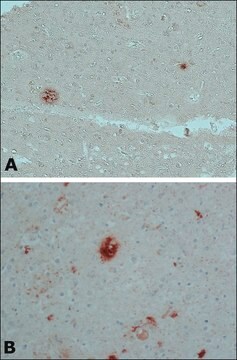
Tau-383 belongs to the neuronal microtubule-associated protein family and is found in the axons of the CNS (central nervous system). It is encoded by the MAPT (microtubule associated protein tau) gene on chromosome 17q21 and exists in the form of 6 isoforms produced by the alternative splicing of m-RNA transcripts of MAPT (microtubule associated protein tau).
Tau-383 belongs to the neuronal microtubule-associated protein family and is found in the axons of the CNS (central nervous system). It is encoded by the MAPT (microtubule associated protein tau) gene on chromosome 17q21 and exists in the form of 6 isoforms produced by the alternative splicing of m-RNA transcripts of MAPT (microtubule associated protein tau).
Application
The product can be utilized for systemic identification of Phosphorylation sites in microtubule-associated protein tau by using checkpoint kinases Chk1 and Chk2 in vitro.
Biochem/physiol Actions
Microtubule-associated protein tau is mainly involved in promoting the assembly and stability of microtubules in axons. The N terminal domain of tau protein is responsible for many important functions including regulation of microtubule dynamics, and spacing between microtubules and cell compartments. While the proline rich domain of the tau protein plays a key role in neuronal signaling, functioning, and maintenance of the cytoskeleton. The interaction between tau and the microtubules plays a crucial role in regulating cell signaling, synaptic plasticity, and genomic stability. Thus, an impaired interaction between Tau and microtubules leads to the development of several neurodegenerative diseases.
Isoform of Tau, variant 0N4R, having 4 microtubule binding repeats (R) and no amino terminal inserts (N)
Reconstitution
Lyophilized from MES, pH 6.8, containing NaCl and EGTA. When reconstituted in water to a protein concentration of 1 mg/mL, the resulting buffer will have ~50 mM MES, pH 6.8, 100 mM NaCl, and 0.5 mM EGTA.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Jhoana Mendoza et al.
Journal of proteome research, 12(6), 2654-2665 (2013-04-05)
Hyperphosphorylation of microtubule-associated protein tau is thought to contribute to Alzheimer's disease (AD) pathogenesis. We previously showed that DNA damage-activated cell cycle checkpoint kinases Chk1 and Chk2 phosphorylate tau at an AD-related site and enhance tau toxicity, suggesting potential roles
A Himmler et al.
Molecular and cellular biology, 9(4), 1381-1388 (1989-04-01)
Tau proteins consist of a family of proteins, heterogeneous in size, which associate with microtubules in vivo and are induced during neurite outgrowth. In humans, tau is one of the major components of the pathognomonic neurofibrillary tangles in Alzheimer's disease
Jesus Avila et al.
Physiological reviews, 84(2), 361-384 (2004-03-27)
The morphology of a neuron is determined by its cytoskeletal scaffolding. Thus proteins that associate with the principal cytoskeletal components such as the microtubules have a strong influence on both the morphology and physiology of neurons. Tau is a microtubule-associated
M Goedert et al.
Neuron, 3(4), 519-526 (1989-10-01)
We have determined the sequences of isoforms of human tau protein, which differ from previously reported forms by insertions of 29 or 58 amino acids in the amino-terminal region. Complementary DNA cloning shows that the insertions occur in combination with
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico