SRP3036
Fas Ligand human
recombinant, expressed in CHO cells, ≥95% (SDS-PAGE), ≥95% (HPLC), suitable for cell culture
Sinónimos:
APTL, Apo I Ligand, CD95L, TNFSF6, soluble Fas Ligand (sFasL)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Productos recomendados
biological source
human
recombinant
expressed in CHO cells
assay
≥95% (HPLC)
≥95% (SDS-PAGE)
form
lyophilized
potency
≤10.0 ng/mL
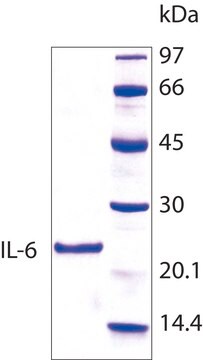
mol wt
17.9 kDa
packaging
pkg of 10 μg
technique(s)
cell culture | mammalian: suitable
impurities
<0.1 EU/μg endotoxin, tested
color
white to off-white
suitability
suitable for molecular biology
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... FASLG(356)
General description
Fas Ligand (FasL) is a member of the TNF (tumor necrosis factor) superfamily that is expressed on the cell surface of activated T cells. It can be present as soluble form in the circulation or membrane bound form in cells. Recombinant human soluble Fas Ligand is a 17.9kDa protein comprising the TNF homologous region of FasL and contains an 8 residue N-terminal His-Tag. Both human and murine sFasL are fully active on human and murine cells.
Application
Fas ligand human has been used to induce apoptosis in T-cell lymphoma-derived HuT78 cells and jurkat cells.
Biochem/physiol Actions
Binding of Fas Ligand (FasL) to Fas receptor triggers apoptosis in Fas-bearing cells. FasL has the ability to kill T cells and activated B cells which leads to down-regulation of the immune response. The mechanism of Fas induced apoptosis involves recruitment of pro-caspase 8 through an adaptor molecule called FADD (Fas-Associated protein with death domain) followed by processing of the pro-enzyme to active forms. These active caspases then cleave various cellular substrates leading to the eventual cell death. FasL is also involved in AGE (advanced glycation end-product)-mediated apoptosis in human retinal ARPE-19 cells, suggesting its role in diabetic retinopathy. Changes in the activity of FasL suppresses normal apoptosis, leading to abnormal survival and growth of tumor cells. Mutations in the FasL gene causes autoimmune lymphoproliferative syndrome.
Sequence
HHHHHHHHPS PPPEKKELRK VAHLTGKSNS RSMPLEWEDT YGIVLLSGVK YKKGGLVINE TGLYFVYSKV YFRGQSCNNL PLSHKVYMRN SKYPQDLVMM EGKMMSYCTT GQMWARSSYL GAVFNLTSAD HLYVNVSELS LVNFEESQTF FGLYKL
Physical form
Lyophilized with no additives.
Reconstitution
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein (example 0.1% BSA) and store in working aliquots at -20°C to -80°C.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Fas ligand mediates activation-induced cell death in human T lymphocytes.
Alderson MR, et al.
The Journal of Experimental Medicine, 71-77 (1995)
The molecular architecture of the TNF superfamily.
Bodmer JL, et al.
Trends in Biochemical Sciences, 27, 19-26 (2002)
The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis.
Powell WC, et al.
Current Biology, 9, 1441-1447 (1999)
Karthik M Kodigepalli et al.
Cell cycle (Georgetown, Tex.), 16(2), 179-188 (2016-12-09)
Sterile α motif and HD domain-containing protein 1 (SAMHD1) is a mammalian dNTP hydrolase (dNTPase) that regulates intracellular dNTP balance. We have previously reported that SAMHD1 mRNA and protein levels are significantly downregulated in CD4
D H Lynch et al.
Immunity, 1(2), 131-136 (1994-05-01)
The gene for the mouse Fas ligand was cloned and its chromosomal position determined. Fasl was tightly linked to gld (no crossovers in 567 meiotic events) on mouse chromosome 1 and closely linked with a novel member of the same
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico