SML1779
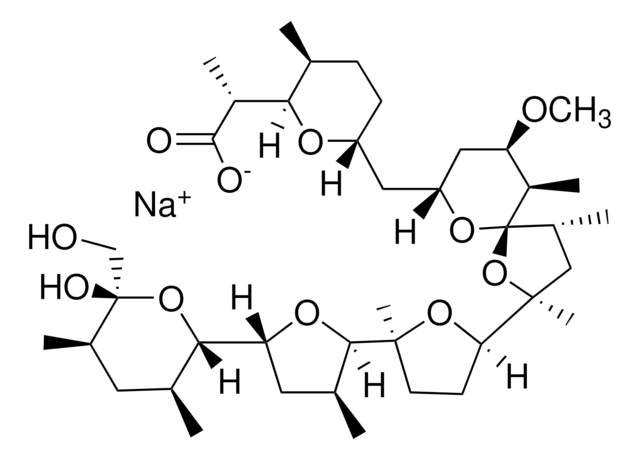
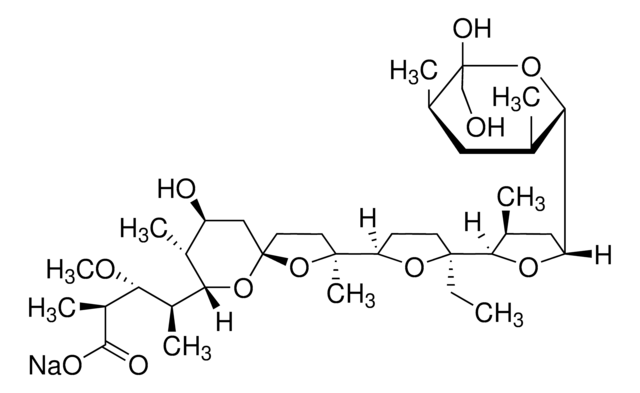
Nigericin sodium salt
from Streptomyces hygroscopicus, ≥98% (HPLC), solution, polyether ionophore
Sinónimos:
3B2-6379, Antibiotic K178, Antibiotic X464, Azalomycin M, HE331800, Helexin C, Polyetherin A, sodium;2-[(3S,6R)-6-[[(5R,6R,7R,9R)-2-[5-[(3S,5R)-5-[(2S,3S,5R,6R)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyloxan-2-yl]-3-methyloxolan-2-yl]-5-methyloxolan-2-yl]-7-methoxy-2,4,6-trimethyl-1,10-dioxaspiro[4.5]decan-9-yl]methyl]-3-methyloxan-2-yl]propanoate
About This Item
Productos recomendados
product name
Nigericin sodium salt Ready Made Solution, 5 mg/mL (DMSO:ethanol 1:1)
biological source
Streptomyces hygroscopicus
Quality Level
form
solution
concentration
5 mg/mL (DMSO:ethanol 1:1)
antibiotic activity spectrum
Gram-positive bacteria
mode of action
cell membrane | interferes
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C40H68O11.Na/c1-21-11-12-28(46-33(21)26(6)36(42)43)17-29-18-30(45-10)27(7)40(48-29)25(5)19-38(9,51-40)32-13-14-37(8,49-32)35-23(3)16-31(47-35)34-22(2)15-24(4)39(44,20-41)50-34;/h21-35,41,44H,11-20H2,1-10H3,(H,42,43);/q;+1/p-1/t21-,22-,23-,24+,25?,26?,27+,28+,29+,30+,31+,32?,33?,34-,35?,37?,38?,39-,40+;/m0./s1
InChI key
MOYOTUKECQMGHE-KKCUGXASSA-M
Categorías relacionadas
Biochem/physiol Actions
Nigericin kills bacteria by facilitating the diffusion of ions across membranes.
Low concentration (0.5 μM) of Nigericin rapidly decreases pHi, causing stimulation of PG production 1.5- to 2-fold in cerebral microvascular endothelial cells and arresting of DNA synthesis in Erlich acites carcinoma cells. Treatment of Hela cells, after entry of poliovirus, with nigericin, prevents the inhibition of host protein synthesis by poliovirus. Nigericin is also widely used in studies of the consequences of changes in membrane potential in variable systems.
Preparation Note
Storage and Stability
signalword
Warning
hcodes
Hazard Classifications
Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
78.8 °F
flash_point_c
26 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico