N176
Naloxonazine dihydrochloride hydrate
powder, ≥95% (HPLC)
Sinónimos:
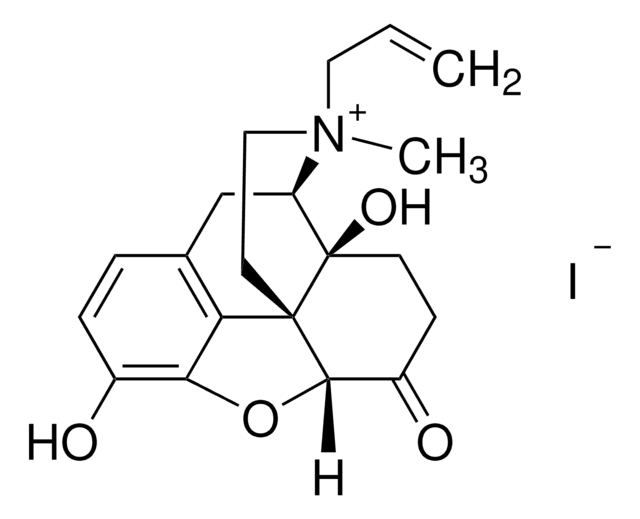
bis[(5α)-4,5-Epoxy-3,14-dihydroxy-17-(2-propenyl)morphinan-6-ylidene]hydrazone hydrate dihydrochloride
About This Item
Productos recomendados
assay
≥95% (HPLC)
form
powder
drug control
regulated under CDSA - not available from Sigma-Aldrich Canada
storage condition
desiccated
color
off-white to light tan
solubility
H2O: >2 mg/mL
storage temp.
−20°C
SMILES string
O.Cl.Cl.CC(=C)CN1CC[C@]23[C@H]4Oc5c(O)ccc(C[C@@H]1[C@]2(O)CC\C4=N/N=C6\CC[C@@]7(O)[C@H]8Cc9ccc(O)c%10O[C@@H]6[C@]7(CCN8CC=C)c9%10)c35
InChI
1S/C39H44N4O6.2ClH.H2O/c1-4-15-42-16-13-36-30-22-5-7-26(44)32(30)48-34(36)24(9-11-38(36,46)28(42)18-22)40-41-25-10-12-39(47)29-19-23-6-8-27(45)33-31(23)37(39,35(25)49-33)14-17-43(29)20-21(2)3;;;/h4-8,28-29,34-35,44-47H,1-2,9-20H2,3H3;2*1H;1H2/b40-24+,41-25+;;;/t28-,29-,34+,35+,36+,37+,38-,39-;;;/m1.../s1
InChI key
FZEFFWGHDWIOOD-VCOBIVQDSA-N
Gene Information
human ... OPRM1(4988)
Biochem/physiol Actions
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico