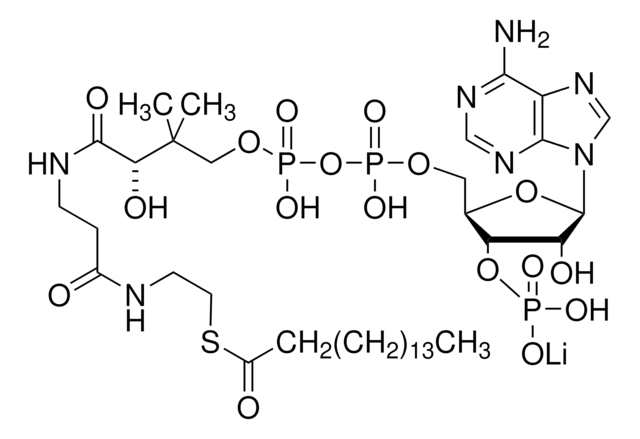
K1502
α-Ketoglutarate Dehydrogenase from porcine heart
buffered aqueous glycerol solution, 0.1-1.0 units/mg protein (Lowry)
Sinónimos:
Multienzyme 2-oxoglutarate dehydrogenase complex
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Número de CAS:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Productos recomendados
form
buffered aqueous glycerol solution
Quality Level
specific activity
0.1-1.0 units/mg protein (Lowry)
foreign activity
pyruvate dehydrogenase ≤20%
shipped in
dry ice
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Research Area: Neuroscience
α-Ketoglutarate dehydrogenase (α-KGDH) is a multienzyme complex localized to the mitochondria. This integrated enzyme is made up of many units of thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide dehydrogenase (E3), and dihydrolipoamide succinyl transferase (E2).
α-Ketoglutarate dehydrogenase (α-KGDH) is a multienzyme complex localized to the mitochondria. This integrated enzyme is made up of many units of thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide dehydrogenase (E3), and dihydrolipoamide succinyl transferase (E2).
Application
α-Ketoglutarate Dehydrogenase from the porcine heart has been used:
- to study the reversal of nitration by glutathione (GSH) in peroxynitrite-treated cells
- to measure its activity by Spectramax M5 microplate spectrofluorimeter using heart mitochondria
- as a positive control to evaluate its activity in by Spectramax GEMINI EM fluorescence microplate reader using mice neurons
Biochem/physiol Actions
α-Ketoglutarate dehydrogenase (α-KGDH) is a key enzyme of bioenergetic processes and a controlling unit of metabolic flux through the Krebs cycle or tricarboxylic acid (TCA) cycle. It catalyzes the oxidative decarboxylation of α-ketoglutarate (KG) to succinyl-CoA by releasing reduced nicotinamide adenine dinucleotide (NADH). It is the rate-limiting reaction of the TCA cycle. This reaction contributes to the electrons of the respiratory chain and requires thiamine pyrophosphate as a cofactor. The reduction of NAD (nicotinamide adenine dinucleotide) is observed to determine its reaction rate. α-KGDH from porcine has an optimum pH range of 6.6–7.4. This enzyme is inhibited by oxidative stress and results in a metabolic deficiency. However, α-KGDH is also known to produce reactive oxygen species (ROS) leading to oxidative stress. Defective or limited levels of α-KGDH cause several neurodegenerative diseases such as Alzheimer′s disease.
α-Ketoglutarate dehydrogenase is most responsive to alterations in the tumor microenvironment and contributes to the adaptive metabolic response in cancer. Inhibiting α-ketoglutarate dehydrogenase counteracts lung metastasis associated with breast cancer.
Quality
May contain traces of polyethylene glycol.
Unit Definition
One unit will convert 1.0 μmole of β-NAD to β-NADH per min at pH 7.4 at 30 °C in the presence of saturating levels of coenzyme A.
Physical form
Supplied as a 50% glycerol solution containing ~9 mg per mL bovine serum albumin, 30% sucrose, 1.5 mM EDTA, 1.5 mM EGTA, 1.5 mM 2-mercaptoethanol, 0.3% TRITON™ X-100, 0.003% sodium azide, and 15 mM potassium phosphate, pH 6.8.
Legal Information
Triton is a trademark of The Dow Chemical Company or an affiliated company of Dow
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 3
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Wagner L Araújo et al.
The Plant cell, 24(6), 2328-2351 (2012-07-04)
Transgenic tomato (Solanum lycopersicum) plants expressing a fragment of the gene encoding the E1 subunit of the 2-oxoglutarate dehydrogenase complex in the antisense orientation and exhibiting substantial reductions in the activity of this enzyme exhibit a considerably reduced rate of
Tristan Wagner et al.
Chemistry & biology, 18(8), 1011-1020 (2011-08-27)
The α-ketoglutarate dehydrogenase (KDH) complex is a major regulatory point of aerobic energy metabolism. Mycobacterium tuberculosis was reported to lack KDH activity, and the putative KDH E1o component, α-ketoglutarate decarboxylase (KGD), was instead assigned as a decarboxylase or carboligase. Here
Shuyi Zhang et al.
Science (New York, N.Y.), 334(6062), 1551-1553 (2011-12-17)
It is generally accepted that cyanobacteria have an incomplete tricarboxylic acid (TCA) cycle because they lack 2-oxoglutarate dehydrogenase and thus cannot convert 2-oxoglutarate to succinyl-coenzyme A (CoA). Genes encoding a novel 2-oxoglutarate decarboxylase and succinic semialdehyde dehydrogenase were identified in
Casey L Quinlan et al.
The Journal of biological chemistry, 289(12), 8312-8325 (2014-02-12)
Several flavin-dependent enzymes of the mitochondrial matrix utilize NAD(+) or NADH at about the same operating redox potential as the NADH/NAD(+) pool and comprise the NADH/NAD(+) isopotential enzyme group. Complex I (specifically the flavin, site IF) is often regarded as
Gary E Gibson et al.
Journal of neurochemistry, 134(1), 86-96 (2015-03-17)
Reversible post-translation modifications of proteins are common in all cells and appear to regulate many processes. Nevertheless, the enzyme(s) responsible for the alterations and the significance of the modification are largely unknown. Succinylation of proteins occurs and causes large changes
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico