I8250
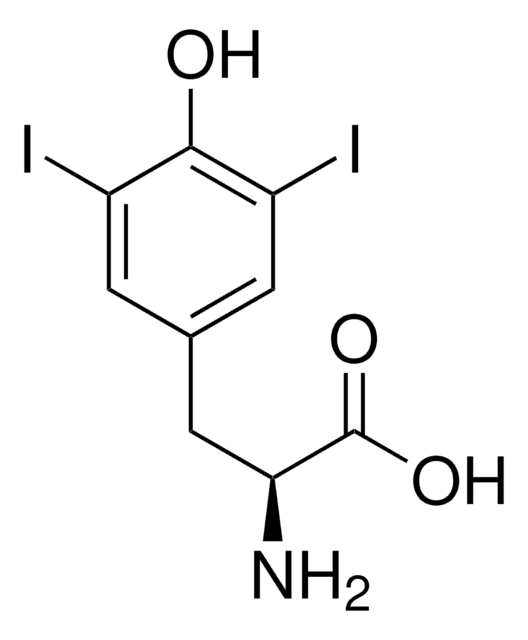
3-Iodo-L-tyrosine
Sinónimos:
3-Monoiodo-L-tyrosine
About This Item
Productos recomendados
Quality Level
impurities
~5% tyrosine
mp
210 °C (dec.) (lit.)
solubility
dilute aqueous acid: soluble
storage temp.
−20°C
SMILES string
N[C@@H](Cc1ccc(O)c(I)c1)C(O)=O
InChI
1S/C9H10INO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)/t7-/m0/s1
InChI key
UQTZMGFTRHFAAM-ZETCQYMHSA-N
Gene Information
human ... TH(7054)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico