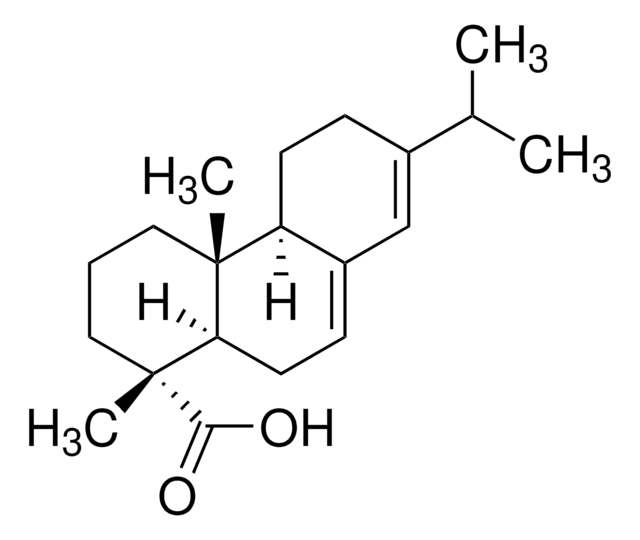
I6783
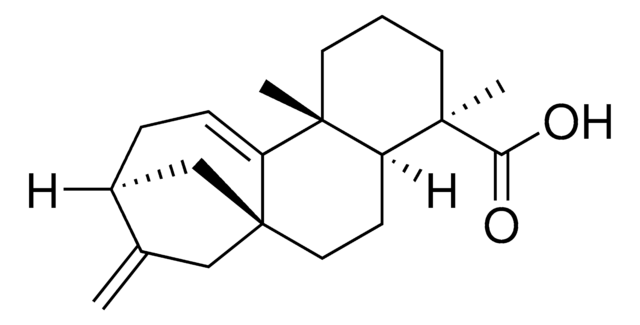
Isopimaric Acid
≥98% (GC), powder
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C20H30O2
Número de CAS:
Peso molecular:
302.45
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.77
Productos recomendados
Quality Level
assay
≥98% (GC)
form
powder
color
white
solubility
DMSO: 25 mg/mL
storage temp.
−20°C
SMILES string
[H][C@]12CC[C@@](C)(CC1=CC[C@@]3([H])[C@@](C)(CCC[C@]23C)C(O)=O)C=C
InChI
1S/C20H30O2/c1-5-18(2)12-9-15-14(13-18)7-8-16-19(15,3)10-6-11-20(16,4)17(21)22/h5,7,15-16H,1,6,8-13H2,2-4H3,(H,21,22)/t15-,16+,18-,19+,20+/m0/s1
InChI key
MXYATHGRPJZBNA-KRFUXDQASA-N
General description
Isopimaric acid, a resin acid produced by conifer trees, is a tricyclic diterpene compound. High levels of isopimaric acid in the hepatocytes of the rainbow trout may impair calcium signalling machinery. Isopimaric acid is a potential big potassium (BK) channel opener.
Biochem/physiol Actions
Potent opener of large conductance calcium activated K+ (BK) channels.
Features and Benefits
This compound is featured on the Potassium Channels page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Neil C Henney et al.
American journal of physiology. Cell physiology, 297(6), C1397-C1408 (2009-09-25)
The pharmacology of the large-conductance K(+) (BK) channel in human osteoblasts is not well defined, and its role in bone is speculative. Here we assess BK channel properties in MG63 cells and primary human osteoblasts and determine whether pharmacological modulation
Eileen Smith et al.
Phytotherapy research : PTR, 19(6), 538-542 (2005-08-23)
The diterpene isopimaric acid was extracted from the immature cones of Pinus nigra (Arnold) using bioassay-guided fractionation of a crude hexane extract. Isopimaric acid was assayed against multidrug-resistant (MDR) and methicillin-resistant Staphylococcus aureus (MRSA). The minimum inhibitory concentrations (MIC) were
Yuji Imaizumi et al.
Molecular pharmacology, 62(4), 836-846 (2002-09-19)
Effects of pimaric acid (PiMA) and eight closely related compounds on large-conductance K(+) (BK) channels were examined using human embryonic kidney (HEK) 293 cells, in which either the alpha subunit of BK channel (HEKBKalpha) or both alpha and beta1 (HEKBKalphabeta1)
Dawn E Hall et al.
Plant physiology, 161(2), 600-616 (2013-02-02)
Diterpene resin acids (DRAs) are major components of pine (Pinus spp.) oleoresin. They play critical roles in conifer defense against insects and pathogens and as a renewable resource for industrial bioproducts. The core structures of DRAs are formed in secondary
Isolation and characterization of isopimaric acid-degrading bacteria from a sequencing batch reactor.
Wilson AE, et al.
Applied and Environmental Microbiology, 62(9), 3146-3151 (1996)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico