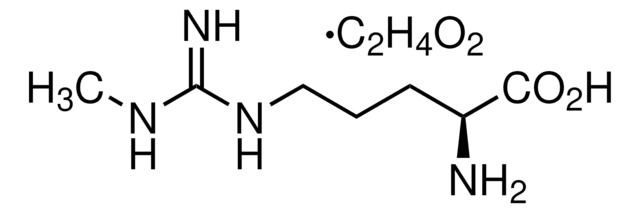
H7278
NG-Hydroxy-L-arginine acetate salt
Sinónimos:
NOHA acetate salt
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H14N4O3 · C2H4O2
Número de CAS:
Peso molecular:
250.25
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.77
Productos recomendados
assay
≥98% (TLC)
Quality Level
form
powder
mp
204 °C
solubility
acetic acid: 10 mg/mL, clear, colorless to faintly yellow
storage temp.
2-8°C
SMILES string
CC(O)=O.N[C@@H](CCCNC(=N)NO)C(O)=O
InChI
1S/C6H14N4O3.C2H4O2/c7-4(5(11)12)2-1-3-9-6(8)10-13;1-2(3)4/h4,13H,1-3,7H2,(H,11,12)(H3,8,9,10);1H3,(H,3,4)/t4-;/m0./s1
InChI key
VYMCYRPQICLHKC-WCCKRBBISA-N
Biochem/physiol Actions
Intermediate in the conversion of arginine to NO and citrulline by NO synthase.
NG-Hydroxy-L-arginine acts as a physiological inhibitor of arginase.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
The inhibition of arginase by N?-hydroxy-L-arginine controls the growth of Leishmania inside macrophages
Iniesta V, et al.
The Journal of Experimental Medicine, 193(6), 777-784 (2001)
F Daghigh et al.
Biochemical and biophysical research communications, 202(1), 174-180 (1994-07-15)
NG-hydroxy-L-arginine, an intermediate in the biosynthesis of nitric oxide (NO), has been found to be a uniquely potent competitive inhibitor of rat liver arginase. Among previously reported inhibitors of arginase and the eight arginine analogs tested herein, only NG-hydroxy-L-arginine was
K L Campos et al.
The Journal of biological chemistry, 270(4), 1721-1728 (1995-01-27)
The nitric oxide synthase-catalyzed conversion of L-arginine to L-citrulline and nitric oxide is known to be the sum of two partial reactions: oxygenation of arginine to N-hydroxyarginine, followed by oxygenation of N-hydroxyarginine to citrulline and nitric oxide. Whereas the conversion
P Klatt et al.
The Journal of biological chemistry, 268(20), 14781-14787 (1993-07-15)
Brain NO (nitric oxide) synthase contains FAD, FMN, heme, and tetrahydrobiopterin as prosthetic groups and represents a multi-functional oxidoreductase catalyzing oxidation of L-arginine to NO and L-citrulline, formation of H2O2, and reduction of cytochrome c. We show that substrate analogues
R A Pufahl et al.
Biochemistry, 34(6), 1930-1941 (1995-02-14)
The ability of murine macrophage nitric oxide synthase (NOS) to utilize peroxides in place of O2 and NADPH was investigated using hydrogen peroxide (H2O2), tert-butylhydroperoxide, and cumene hydroperoxide with both L-arginine and NG-hydroxy-L-arginine (L-NHA) as substrates. Of the three peroxides
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico