E003256
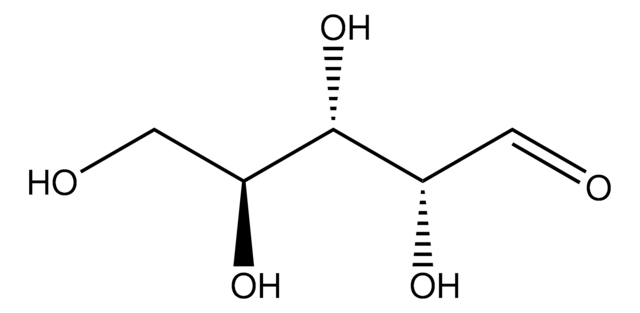
L-(+)-Arabinose
≥99% (GC)
Sinónimos:
Aldehydo-L-arabinose
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H10O5
Número de CAS:
Peso molecular:
150.13
Beilstein/REAXYS Number:
1723085
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
Productos recomendados
Quality Level
assay
≥99% (GC)
form
powder
optical activity
[α]/D +103 to +105°(lit.)
color
white
mp
160-163 °C (lit.)
solubility
water: 100 mg/mL, clear, colorless
SMILES string
OC[C@H](O)[C@H](O)[C@@H](O)C=O
InChI
1S/C5H10O5/c6-1-3(8)5(10)4(9)2-7/h1,3-5,7-10H,2H2/t3-,4-,5+/m0/s1
InChI key
PYMYPHUHKUWMLA-VAYJURFESA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
L-Arabinose is used as a substrate to identify, differentiate and characterize pentose sugar isomerase(s). L-Arabinose is used in the bioproduction of L-ribose.
Biochem/physiol Actions
L-Arabinose is the naturally occurring isomer and is a constituent of plant polysaccharides. Most bacteria contain an inducible arabinose operon that codes for a series of enzymes and transporters that allows L-arabinose to be used as the sole carbon source in microbial culture.
L-(+)-Arabinose is a naturally occurring pentose sugar that has been shown to decrease lipogenesis in rat models due to its ability to inhibit sucrase activity.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
S Osaki et al.
The Journal of nutrition, 131(3), 796-799 (2001-03-10)
L-Arabinose is a natural, poorly absorbed pentose that selectively inhibits intestinal sucrase activity. To investigate the effects of L-arabinose feeding on lipogenesis due to its inhibition of sucrase, rats were fed 0-30 g sucrose/100 g diets containing 0-1 g L-arabinose/100
Koen J T Venken et al.
Nucleic acids research, 36(18), e114-e114 (2008-08-05)
Studying gene function in the post-genome era requires methods to localize and inactivate proteins in a standardized fashion in model organisms. While genome-wide gene disruption and over-expression efforts are well on their way to vastly expand the repertoire of Drosophila
Tobias Bergmiller et al.
BMC microbiology, 11, 118-118 (2011-05-31)
The essential Escherichia coli gene ygjD belongs to a universally conserved group of genes whose function has been the focus of a number of recent studies. Here, we put ygjD under control of an inducible promoter, and used time-lapse microscopy
Naeem Anwar et al.
PloS one, 9(8), e106095-e106095 (2014-08-26)
In Salmonella enterica serovar Typhimurium (S. Typhimurium), biofilm-formation is controlled by the cytoplasmic intracellular small-molecular second messenger cyclic 3', 5'-di- guanosine monophosphate (c-di-GMP) through the activities of GGDEF and EAL domain proteins. Here we describe that deleting either dsbA or
Wei Sun et al.
Infection and immunity, 82(10), 4390-4404 (2014-08-13)
Here, we constructed a Yersinia pseudotuberculosis mutant strain with arabinose-dependent regulated and delayed shutoff of crp expression (araC P(BAD) crp) and replacement of the msbB gene with the Escherichia coli msbB gene to attenuate it. Then, we inserted the asd
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico