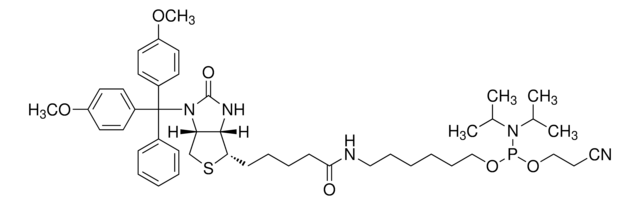
AL11430
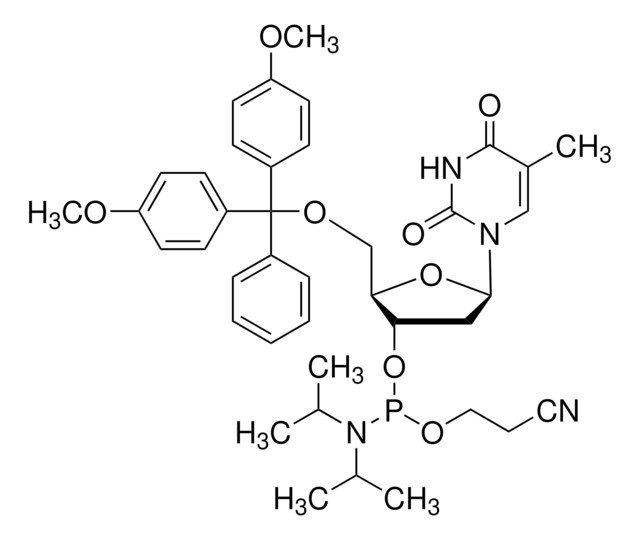
DMT-locA(bz) Phosphoramidite
configured for ABI
About This Item
Productos recomendados
biological source
non-animal source (BSE/TSE no Risk)
Quality Level
product line
Proligo Reagents
assay
≥98.0% (31P-NMR)
≥98.0% (reversed phase HPLC)
form
powder
impurities
≤3 wt. % Residual Solvent Content
<0.4% Water Content (Karl Fischer)
<0.5% Single unspecified Impurity (reversed phase HPLC)
color
white to off-white
solubility
soluble, clear, colorless
absorption
<0.1 in acetonitrile at 0.2 M
suitability
conforms to structure for H-NMR
conforms to structure for LC-MS
compatibility
configured for ABI
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
With the exception of the LNA monomers, LNA synthesis is accomplished with the same reagents as DNA synthesis. LNA phosphoramidites from Merck are diluted with dry acetonitrile, except for locMeC(bz)-phosphoramidite. This phosphoramidite requires the application of a cosolvent to prevent crystallization from the solution on the synthesizer. Dichloromethane or tetrahydrofuran (THF) can be applied as co-solvents with acetonitrile to completely dissolve locMeC(bz)-phosphoramidite.
Features and Benefits
- LNA oligonucleotides are prepared by phosphoramidite chemistry
- Standard DNA synthesizer platforms can be employed. No change is required in the reagents commonly used for DNA synthesis
- To further enhance the hybridization characteristics of LNA, 5-methyl-cytidine is employed instead of cytidine
- LNA monomers are as soluble in acetonitrile as their DNA counterparts (except for the 5-methyl-cytidine derivative, which requires the application of 10-20%, dichloromethane as a co-solvent)
- Mixmer oligonucleotides containing LNA, DNA and/or RNA monomers can be assembled easily
- LNA oligonucleotides with predefined melting temperatures (Tm) can be designed and prepared
Other Notes
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico