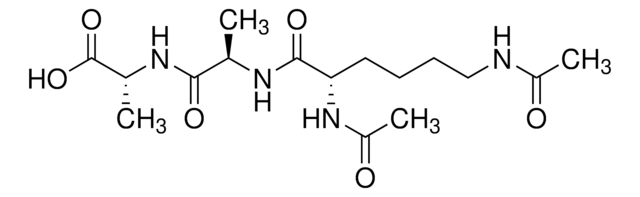
A6950
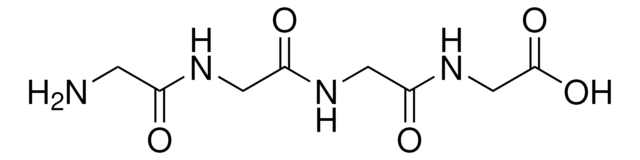
Acetyl-Lys-D-Ala-D-Ala
≥95% (HPLC), powder
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C14H26N4O5
Número de CAS:
Peso molecular:
330.38
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Productos recomendados
Nombre del producto
Acetyl-Lys-D-Ala-D-Ala, ≥95% (HPLC)
assay
≥95% (HPLC)
form
powder
solubility
water: 10 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CC(NC(=O)C(C)NC(=O)C(CCCCN)NC(C)=O)C(O)=O
InChI
1S/C14H26N4O5/c1-8(12(20)17-9(2)14(22)23)16-13(21)11(18-10(3)19)6-4-5-7-15/h8-9,11H,4-7,15H2,1-3H3,(H,16,21)(H,17,20)(H,18,19)(H,22,23)
InChI key
GMSXMADYKTYBCP-UHFFFAOYSA-N
Substrates
Substrate for carboxypeptidase G and DD from Streptomyces albus.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
D H Williams et al.
Science (New York, N.Y.), 280(5364), 711-714 (1998-05-23)
The cooperativity between binding of cell wall precursor analogs (ligands) to and antibiotic dimerization of the clinically important vancomycin group antibiotics was investigated by nuclear magnetic resonance. When dimerization was weak in the absence of a ligand, the increase in
L Varetto et al.
European journal of biochemistry, 162(3), 525-531 (1987-02-02)
Titration of the active-site serine DD-peptidase of Streptomyces R61 shows that formation of acyl enzyme during hydrolysis of the substrate Ac2-L-Lys-D-Ala-D-Ala and enzyme inactivation by the beta-lactam compounds benzylpenicillin, N-acetylampicillin and ampicillin relies on the acidic form of an enzyme's
The structure of an asymmetric dimer relevant to the mode of action of the glycopeptide antibiotics.
P Groves et al.
Structure (London, England : 1993), 2(8), 747-754 (1994-08-15)
Glycopeptide antibiotics of the vancomycin group are of crucial clinical importance in the treatment of methicillin resistant Staphylococcus aureus (MRSA)--the often lethal 'super-bug'--characterized by its resistance to a wide range of antibiotics in common use. The antibiotics exert their physiological
Eric C DiBiasio et al.
Journal of bacteriology, 202(20) (2020-08-12)
Uropathogenic Escherichia coli (UPEC) is the leading cause of human urinary tract infections (UTIs), and many patients experience recurrent infection after successful antibiotic treatment. The source of recurrent infections may be persistent bacterial reservoirs in vivo that are in a
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico