186325
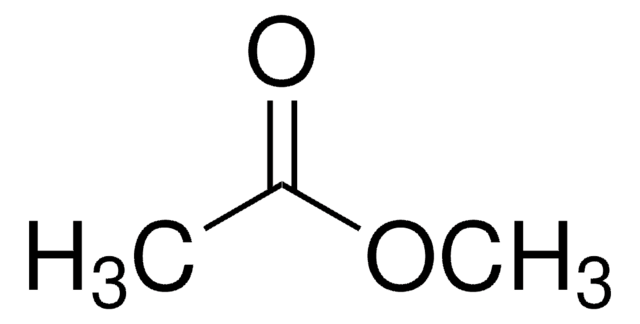
Methyl acetate
ReagentPlus®, 99%
About This Item
Productos recomendados
grade
reagent
Quality Level
vapor density
2.55 (vs air)
vapor pressure
165 mmHg ( 20 °C)
product line
ReagentPlus®
assay
99%
form
liquid
autoignition temp.
936 °F
expl. lim.
16 %
dilution
(for general lab use)
refractive index
n20/D 1.361 (lit.)
bp
57-58 °C (lit.)
mp
−98 °C (lit.)
density
0.934 g/mL at 25 °C
SMILES string
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
InChI key
KXKVLQRXCPHEJC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- As acyl acceptor in the preparation of biodiesel.
- Synthesis of ethanol.
- Preparation of n-butyl acetate, via transesterification reaction with n-butanol in the presence of acidic catalysts.
- acetic anhydride
- methyl acrylate
- vinyl acetate
- ethyl amide
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
target_organs
Central nervous system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
8.6 °F - closed cup
flash_point_c
-13 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico