PHR1620
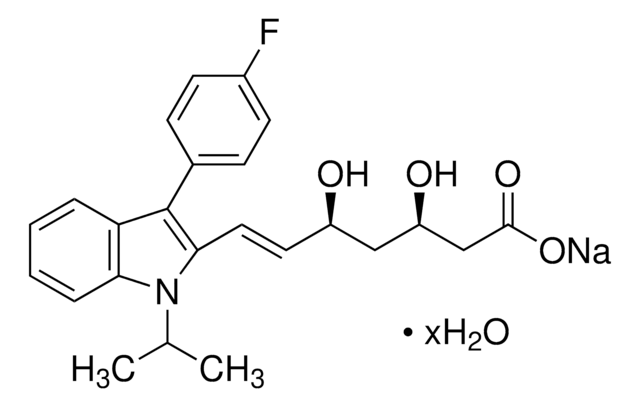
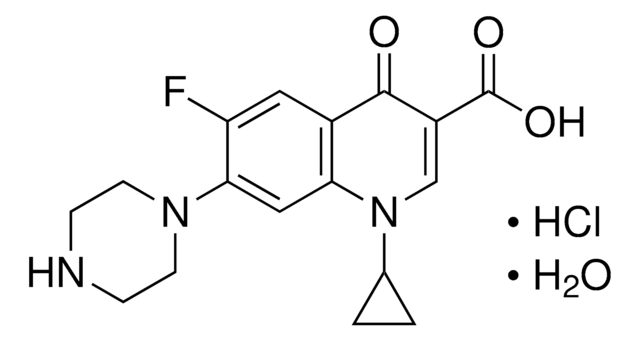
Fluvastatin sodium
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
(3R,5S,6E)-rel-7-[3-(4-Fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid monosodium salt
About This Item
Productos recomendados
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. Y0001082
traceable to USP 1285931
API family
fluvastatin
CofA
current certificate can be downloaded
packaging
ampule of 1 × 1 g
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
[Na+].CC(C)n1c(\C=C\[C@H](O)C[C@H](O)CC([O-])=O)c(-c2ccc(F)cc2)c3ccccc13
InChI
1S/C24H26FNO4.Na/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);/q;+1/p-1/b12-11+;/t18-,19-;/m0./s1
InChI key
ZGGHKIMDNBDHJB-RPQBTBOMSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Footnote
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico