L0702000
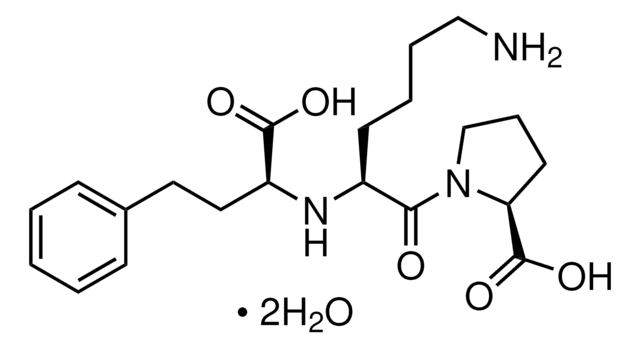
Lisinopril dihydrate
European Pharmacopoeia (EP) Reference Standard
Sinónimos:
Lisinopril, (S)-1-[N2-(1-carboxy-3-phenylpropyl)-lysyl-proline dihydrate, MK-521
About This Item
Productos recomendados
grade
pharmaceutical primary standard
API family
lisinopril
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
[H]O[H].[H]O[H].NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N2CCC[C@H]2C(O)=O
InChI
1S/C21H31N3O5.2H2O/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15;;/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29);2*1H2/t16-,17-,18-;;/m0../s1
InChI key
CZRQXSDBMCMPNJ-ZUIPZQNBSA-N
Gene Information
human ... ACE(1636)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
Packaging
Other Notes
Related product
signalword
Danger
hcodes
Hazard Classifications
Repr. 1A - STOT RE 2
target_organs
Kidney
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico