P-008
Phenobarbital solution
1 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Sinónimos:
5-Ethyl-5-phenylbarbituric acid solution
About This Item
Productos recomendados
grade
certified reference material
form
liquid
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
concentration
1 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
-10 to -25°C
SMILES string
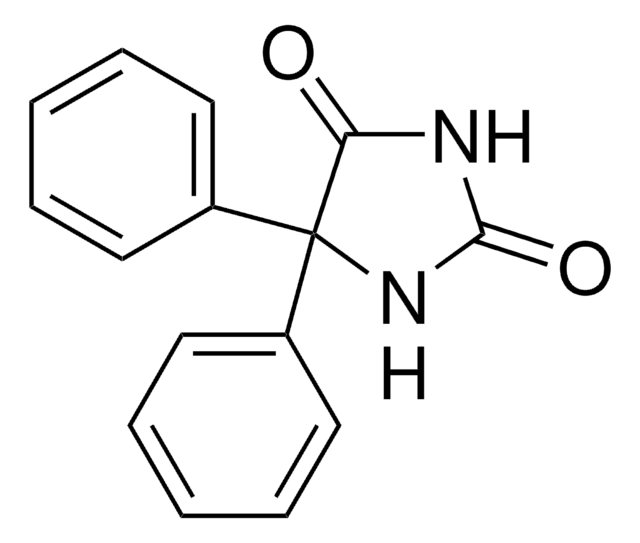
CCC1(C(=O)NC(=O)NC1=O)c2ccccc2
InChI
1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17)
InChI key
DDBREPKUVSBGFI-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Fabrication of a Surface Molecularly Imprinted Polymer Membrane: Phenobarbital is utilized in the development of molecularly imprinted polymer membranes for the selective separation and extraction of various pharmaceutical compounds, enhancing the precision and efficiency of drug monitoring in clinical settings. This application leverages the specific binding properties of Phenobarbital to improve sample preparation techniques for complex biological matrices (Zhao et al., 2024).
- An Isotope Dilution-Liquid Chromatography-Tandem Mass Spectrometry-Based Reference Measurement Procedure: Phenobarbital′s role in establishing reference measurement procedures underscores its importance in ensuring the accuracy and reliability of drug quantification in clinical laboratories. This application supports the standardization of laboratory measurements, critical for patient care and therapeutic monitoring (Schierscher et al., 2024).
Recommended products
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
HPLC Analysis of Barbiturates in Serum on Discovery® C18 after SPE using Discovery® DSC-18Lt
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico