M-136
Methotrexate solution
1.0 mg/mL in methanol with 0.1N NaOH, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol with 0.1N NaOH
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
pharmaceutical (small molecule)
format
single component solution
storage temp.
−20°C
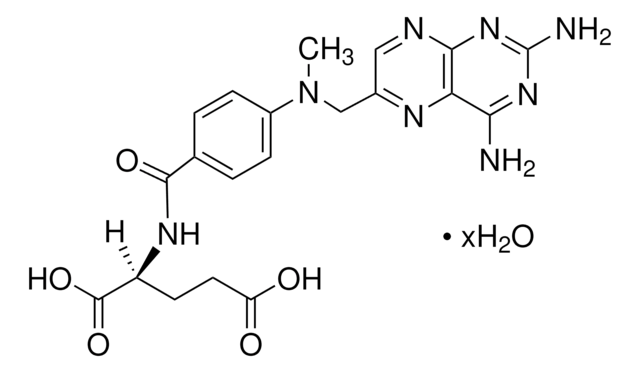
SMILES string
O=C(C1=CC=C(N(C)CC2=NC3=C(N=C2)N=C(N)N=C3N)C=C1)NC(C(O)=O)CCC(O)=O
InChI
1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)
InChI key
FBOZXECLQNJBKD-UHFFFAOYSA-N
General description
Application
- Pharmacokinetic analysis: Methotrexate solution is employed in advanced pharmacokinetic studies using UV-Vis spectrophotometric and colorimetric methods. This application facilitates precise quantification in plasma and tissue, crucial for evaluating drug distribution and effectiveness in cancer treatments (Febrianti et al., 2024).
- Toxicity and safety monitoring: Observational studies utilize Methotrexate solution to investigate root causes of medication errors and manage toxicity in elderly patients. This research supports safer clinical practices by identifying risk factors and improving patient safety protocols (Bisht et al., 2024).
- Nephrotoxicity prevention: Research on Methotrexate solution examines hydration strategies to mitigate nephrotoxicity, enhancing therapeutic outcomes. This study is pivotal in optimizing Methotrexate use in clinical settings, ensuring higher safety and efficacy for patients undergoing chemotherapy (Hasanpour et al., 2024).
Legal Information
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - Met. Corr. 1 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
51.8 °F - closed cup
flash_point_c
11 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico