D-915
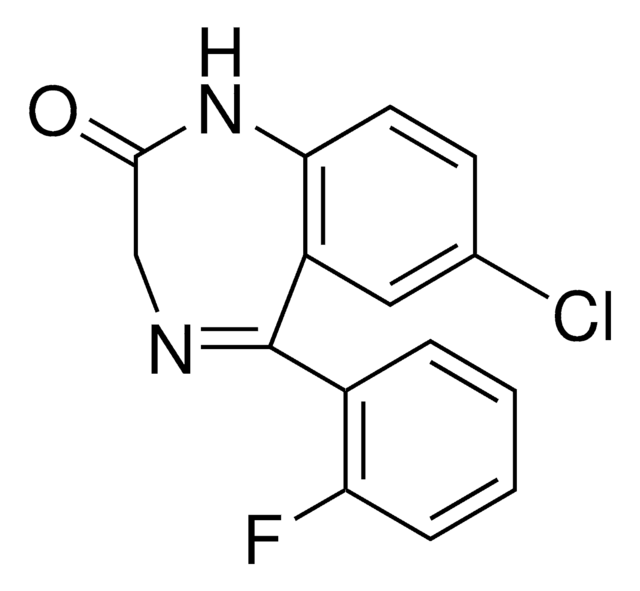
Desalkylflurazepam solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
Fc1ccccc1C2=NCC(=O)Nc3ccc(Cl)cc23
InChI
1S/C15H10ClFN2O/c16-9-5-6-13-11(7-9)15(18-8-14(20)19-13)10-3-1-2-4-12(10)17/h1-7H,8H2,(H,19,20)
InChI key
UVCOILFBWYKHHB-UHFFFAOYSA-N
General description
Legal Information
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico