Q1603
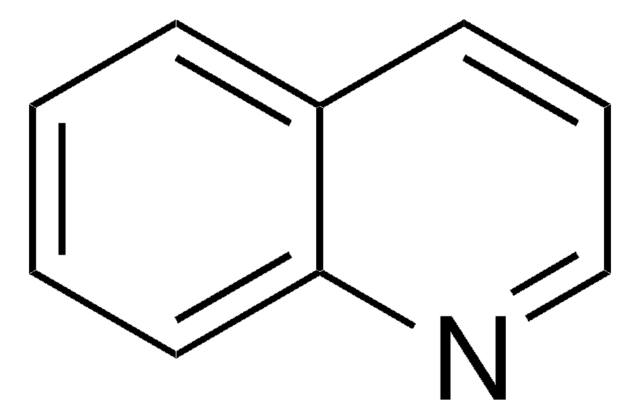
Quinoxaline
≥98%
Sinónimos:
1,4-Benzodiazine, Benzo[a]pyrazine, Benzopyrazine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6N2
Número de CAS:
Peso molecular:
130.15
Beilstein/REAXYS Number:
109351
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98%
bp
220-223 °C (lit.)
mp
29-32 °C (lit.)
density
1.124 g/mL at 25 °C (lit.)
SMILES string
c1ccc2nccnc2c1
InChI
1S/C8H6N2/c1-2-4-8-7(3-1)9-5-6-10-8/h1-6H
InChI key
XSCHRSMBECNVNS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Mark T Bilodeau et al.
Bioorganic & medicinal chemistry letters, 18(11), 3178-3182 (2008-05-16)
A series of naphthyridine and naphthyridinone allosteric dual inhibitors of Akt1 and 2 have been developed. These compounds have been optimized to have potent dual activity against the activated kinase as well as the activation of Akt in cells. One
Wei Zhang et al.
Journal of agricultural and food chemistry, 67(22), 6350-6358 (2019-05-16)
α-Dicarbonyls are reactive intermediates formed during Maillard reactions and carbohydrate degradation. The formation of seven α-dicarbonyls was characterized in solutions containing dairy related carbohydrates (galactose, glucose, lactose, and galacto-oligosaccharides (GOS)) during incubations at 40 and 50 °C with and without
Neslihan Göncüoğlu Taş et al.
Journal of agricultural and food chemistry, 67(1), 415-424 (2018-12-12)
This study investigated the effect of roasting (150 °C for 30 min) and storage (12 months at 4 °C, 25 °C, and 25 °C in vacuum package), conditions of which are generally applied in the industry and markets, on the
F Docobo-Pérez et al.
Antimicrobial agents and chemotherapy, 59(9), 5602-5610 (2015-07-01)
The aim of this study was to improve the understanding of the pharmacokinetic-pharmacodynamic relationships of fosfomycin against extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli strains that have different fosfomycin MICs. Our methods included the use of a hollow fiber infection model with
Aytül Hamzalıoğlu et al.
Food chemistry, 318, 126467-126467 (2020-03-08)
This study aims to investigate in depth the mechanism of acrylamide formation in coffee during roasting. For this purpose, a comprehensive kinetic model including the elementary steps for acrylamide formation was proposed. The changes in sucrose, reducing sugars, free amino
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico