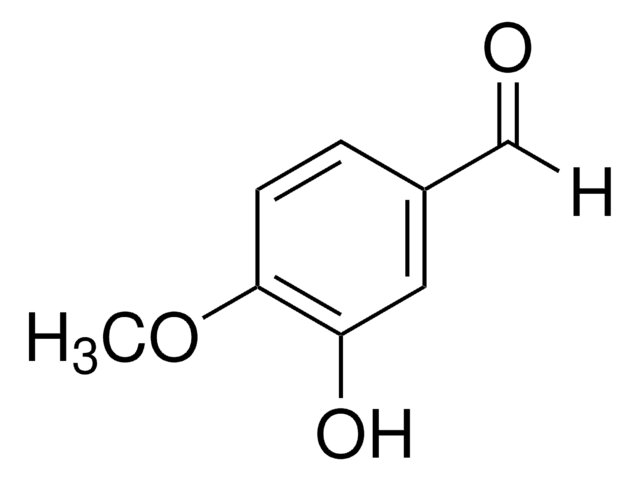
D108405
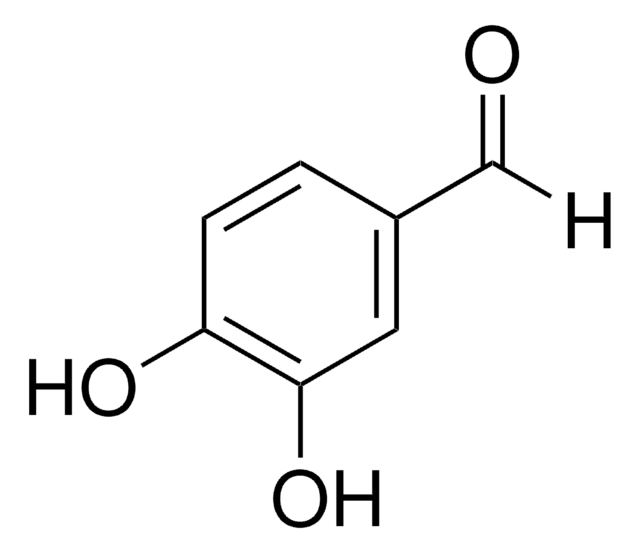
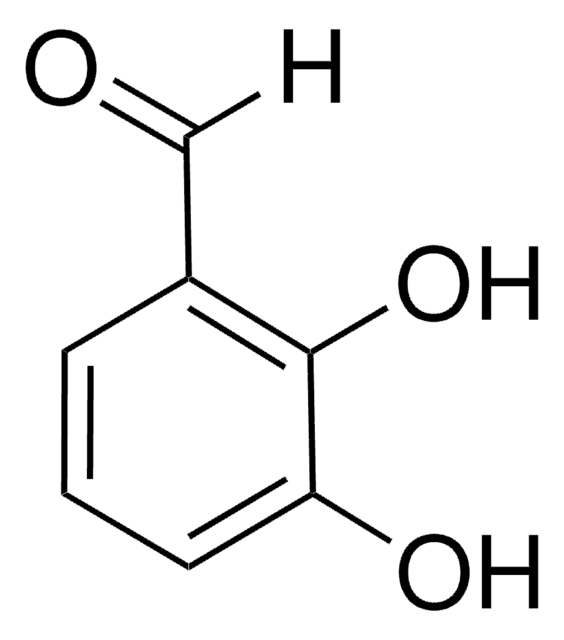
3,4-Dihydroxybenzaldehyde
97%
Sinónimos:
Protocatechualdehyde
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(HO)2C6H3CHO
Número de CAS:
Peso molecular:
138.12
Beilstein/REAXYS Number:
774381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
powder
mp
150-157 °C (lit.)
SMILES string
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
InChI key
IBGBGRVKPALMCQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
3,4-Dihydroxybenzaldehyde can be used as a reactant for the synthesis of:
- Copolymers containing poly(p-phenylenevinylene) chromophore to be used in light-emitting electrochemical cell.
- 2-Arylbenzothiazoles with potential application as anti-cancer agents against human colon cancer cells.
- Variety of thiazolidin-4-one ring systems having antimicrobial activity.
- Bis-Schiff bases of isatins which can be used as antiglycating agents.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthesis of bis-Schiff bases of isatins and their antiglycation activity.
Khan KM, et al.
Bioorganic & Medicinal Chemistry, 17(22), 7795-7801 (2009)
Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials.
Kavitha CV, et al.
Bioorganic & Medicinal Chemistry, 14(7), 2290-2299 (2006)
Synthesis and electroluminescence of novel copolymers containing crown ether spacers.
Sun Q, et al.
Journal of Materials Chemistry, 13(4), 800-806 (2003)
Catriona G Mortimer et al.
Journal of medicinal chemistry, 49(1), 179-185 (2006-01-06)
A series of new 2-phenylbenzothiazoles has been synthesized on the basis of the discovery of the potent and selective in vitro antitumor properties of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (8n; GW 610, NSC 721648). Synthesis of analogues substituted in the benzothiazole ring was achieved
Narsimha Mamidi et al.
The journal of physical chemistry. B, 116(35), 10684-10692 (2012-08-07)
Diacylglycerol (DAG) regulates a broad range of cellular functions including tumor promotion, apoptosis, differentiation, and growth. Thus, the DAG-responsive C1 domain of protein kinase C (PKC) isoenzymes is considered to be an attractive drug target for the treatment of cancer
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| D108405-25G | 4061833558638 |
| D108405-100G | 4061833558621 |
| D108405-5G | 4061833558645 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico