C109800
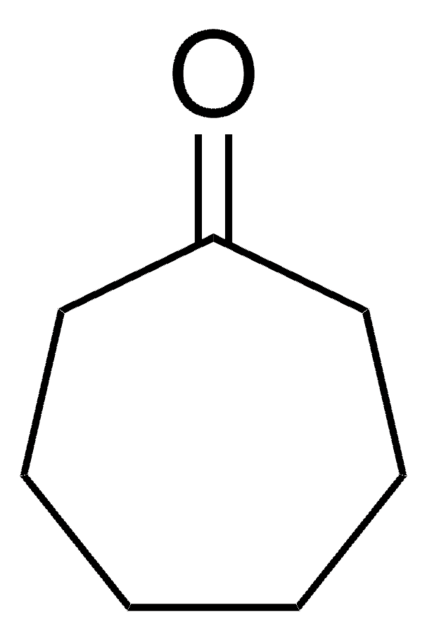
Cyclooctanone
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
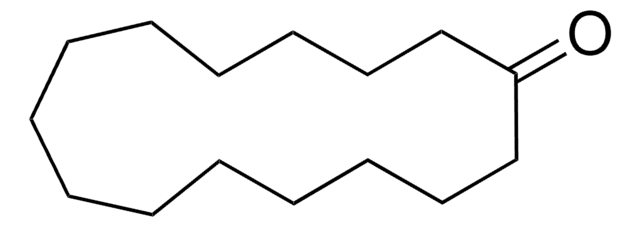
C8H14(=O)
Número de CAS:
Peso molecular:
126.20
Beilstein/REAXYS Number:
1280738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
crystals
bp
195-197 °C (lit.)
mp
32-41 °C (lit.)
density
0.958 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCCCCC1
InChI
1S/C8H14O/c9-8-6-4-2-1-3-5-7-8/h1-7H2
InChI key
IIRFCWANHMSDCG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
165.2 °F
flash_point_c
74 °C
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Vishwakarma Singh et al.
The Journal of organic chemistry, 70(3), 973-981 (2005-01-29)
A new and efficient synthesis of a variety of highly embellished bicyclooctenones having an endo-vinyl moiety and their sigmatropic shifts in ground and excited states leading to a stereoselective route to substituted cis-decalins and diquinane frameworks have been described. Functionalized
M E Krafft et al.
The Journal of organic chemistry, 66(22), 7443-7448 (2001-10-30)
The total synthesis of asteriscanolide (1) has been achieved by taking advantage on an intermolecular Pauson-Khand cycloaddition and a ring-closing metathesis as key bond-forming transformations. The approach incorporates the cyclooctane stereogenic center prior to ring formation. Interestingly, the ring-closing metathesis
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 1898-1905 (1998-01-20)
Construction of the AB-ring system of the taxane framework via an A-ring annulation strategy was demonstrated by base-mediated intramolecular aldol reaction of (Z)-2,2-dimethyl-3-(1-methyl-2-oxopropylidene)cyclooctanone, affording the title compound, 1-hydroxy-8,11,11-trimethylbicyclo[5.3.1]undec-7-en-9-one. A cyclization precursor, the tetra-substituted (Z)-alkene, was prepared from the corresponding cyclooctanone
F E Harvey et al.
Brain research bulletin, 13(4), 541-547 (1984-10-01)
Female mice were reared in observation incubators from day 1 of life for three weeks. During that time they were continuously exposed to the odors of either cyclooctanone, adult male mouse urine or distilled water. The growth rate was temporarily
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 2113-2115 (1998-01-20)
Stereoselective syntheses of omega-(alpha-bromoketo) octanals and nonanal with oxygenated functions and formation of the corresponding eight-membered carbocyclic aldols by subsequent samarium(II)-mediated cyclization are demonstrated. Cyclooctenones deoxygenated at the C2 or C10 position in the taxane framework are prepared by dehydration
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| C109800-25G | 4061833460696 |
| C109800-100G | 4061833460689 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico