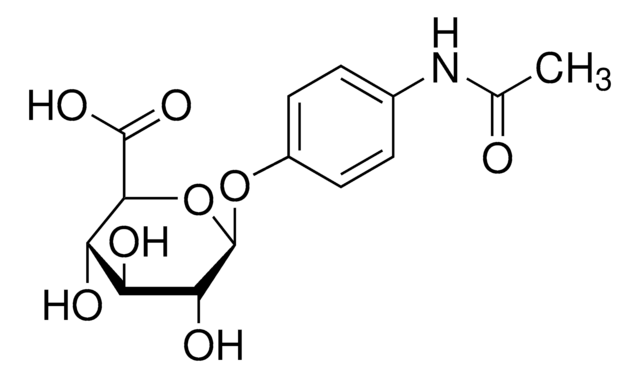
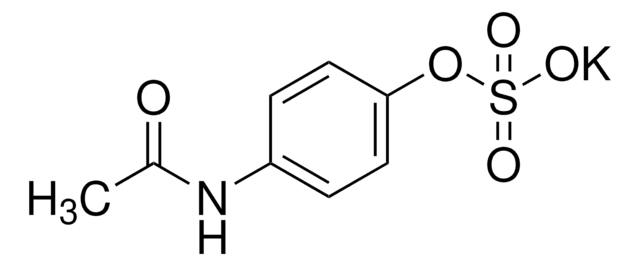
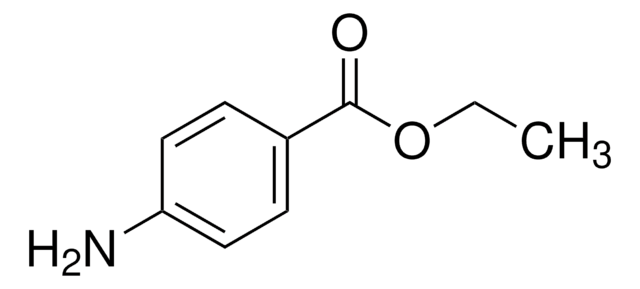
A7300
N-Acetylbenzoquinoneimine
Sinónimos:
N-(4-oxo-1-cyclohexa-2,5-dienylidene)acetamide, N-Acetyl-p-benzo-quinone imine, NAPQI
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H7NO2
Número de CAS:
Peso molecular:
149.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
form
solid
Quality Level
storage temp.
−70°C
SMILES string
CC(=O)\N=C1\C=CC(=O)C=C1
InChI
1S/C8H7NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5H,1H3
InChI key
URNSECGXFRDEDC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Reactant involved in:
- Redox reactions
- Hydrohalogenation
- pH dependent reactions: in acidic media it is hydrolyzed, hydroxylated in alkaline media, and dimerized at intermediate pHs
- Mediation of acetaminophen hepatotoxicity
- Studies to identify the utility of acetaminophen for treating autoimmune disorders
Other Notes
Acetaminophen metabolite that reacts with serum proteins.
Caution
air, moisture and light sensitive
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Angela S Burke et al.
Chemical research in toxicology, 23(7), 1286-1292 (2010-06-29)
Acetaminophen (APAP) toxicity in primary mouse hepatocytes occurs in two phases. The initial phase (0-2 h) occurs with metabolism to N-acetyl-p-benzoquinoneimine which depletes glutathione, and covalently binds to proteins, but little toxicity is observed. Subsequent washing of hepatocytes to remove
Laura P James et al.
Drug metabolism and disposition: the biological fate of chemicals, 37(8), 1779-1784 (2009-05-15)
Acetaminophen (APAP)-induced liver toxicity occurs with formation of APAP-protein adducts. These adducts are formed by hepatic metabolism of APAP to N-acetyl-p-benzoquinone imine, which covalently binds to hepatic proteins as 3-(cystein-S-yl)-APAP adducts. Adducts are released into blood during hepatocyte lysis. We
Amaresh Kumar Sahoo et al.
Nanoscale, 3(10), 4226-4233 (2011-09-08)
Herein, we report the generation of a composite comprised of p-hydroxyacetanilide dimer and Ag nanoparticles (NPs) by reaction of AgNO(3) and p-hydroxyacetanilide. The formation of the composite was established by UV-vis, FTIR and NMR spectroscopy, transmission electron microscopy and X-ray
Hui Ting Chng et al.
Journal of biomolecular screening, 17(7), 974-986 (2012-05-31)
The zebrafish model has been increasingly explored as an alternative model for toxicity screening of pharmaceutical drugs. However, little is understood about the bioactivation of drug to reactive metabolite and phase I and II metabolism of chemical in zebrafish as
Cross-linking of protein molecules by the reactive metabolite of acetaminophen, N-acetyl-p-benzoquinone imine, and related quinoid compounds.
A J Streeter et al.
Advances in experimental medicine and biology, 197, 727-737 (1986-01-01)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico