A71301
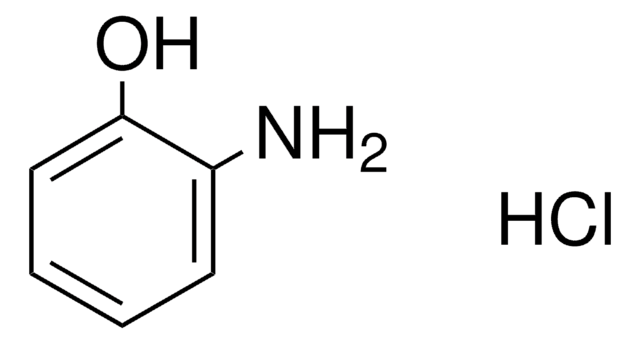
2-Aminophenol
99%
Sinónimos:
(2-Hydroxyphenyl)amine, 1-Hydroxy-2-aminobenzene, 2-Amino-1-hydroxybenzene, 2-Aminophenol, 2-Aminophenyl alcohol, 2-Hydroxyaniline, 2-Hydroxybenzenamine, o-Aminohydroxybenzene, o-Hydroxyaniline, o-Hydroxyphenylamine
About This Item
assay
99%
form
powder
mp
170-175 °C (lit.)
SMILES string
Nc1ccccc1O
InChI
1S/C6H7NO/c7-5-3-1-2-4-6(5)8/h1-4,8H,7H2
InChI key
CDAWCLOXVUBKRW-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- 2-Oxazolidinone derivatives by reacting with β-aminoalcohols in presence of Pd/C-I2 as a catalyst via oxidative cyclocarbonylation.
- Schiff base transition metal(II) complexes with salicylidene-4-aminoantipyrine.
- 2-Arylbenzoxazoles with aldehydes catalyzed by activated carbon in presence of oxygen atmosphere.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Muta. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
334.4 °F - closed cup
flash_point_c
168 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico